The HIV-1 gag p6: a promising target for therapeutic intervention
- PMID: 38263239
- PMCID: PMC10807055
- DOI: 10.1186/s12977-024-00633-2
The HIV-1 gag p6: a promising target for therapeutic intervention
Abstract
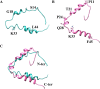
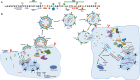
The p6 domain of the Gag precursors (Gag p6) in human immunodeficiency virus type 1 (HIV-1) plays multifunctional roles in the viral life cycle. It utilizes the endosomal sorting complex required for transport (ESCRT) system to facilitate viral budding and release from the plasma membrane through the interactions with the ESCRT-I component tumor susceptibility gene 101 (TSG101) and with the ALG-2 interacting protein X (ALIX). Moreover, Gag p6 contributes to viral replication by a range of posttranslational modifications such as SUMOylation, ubiquitination and phosphorylation. Additionally, Gag p6 also mediates the incorporation of the accessory protein Vpr into virions, thereby promoting Vpr-induced viral replication. However, less attention is focused on Gag p6 as therapeutic intervention. This review focuses on the structures and diverse functions of Gag p6 in viral replication, host cells, and pathogenesis. Additionally, several challenges were also discussed in studying the structure of Gag p6 and its interactions with partners. Consequently, it concludes that the Gag p6 represents an attractive target for the development of antiretroviral drugs, and efforts to develop p6-targeted antiretrovirals are expected to undergo significant growth in the forthcoming years.
Keywords: Antiretrovirals; HIV-1 Gag p6; Viral replication and budding.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Glutamic Acid Residues in HIV-1 p6 Regulate Virus Budding and Membrane Association of Gag.Viruses. 2016 Apr 25;8(4):117. doi: 10.3390/v8040117. Viruses. 2016. PMID: 27120610 Free PMC article.
-
Ubiquitin conjugation to Gag is essential for ESCRT-mediated HIV-1 budding.Retrovirology. 2013 Jul 29;10:79. doi: 10.1186/1742-4690-10-79. Retrovirology. 2013. PMID: 23895345 Free PMC article.
-
Alix-Mediated Rescue of Feline Immunodeficiency Virus Budding Differs from That Observed with Human Immunodeficiency Virus.J Virol. 2020 May 18;94(11):e02019-19. doi: 10.1128/JVI.02019-19. Print 2020 May 18. J Virol. 2020. PMID: 32213612 Free PMC article.
-
Post-Translational Modifications of Retroviral HIV-1 Gag Precursors: An Overview of Their Biological Role.Int J Mol Sci. 2021 Mar 11;22(6):2871. doi: 10.3390/ijms22062871. Int J Mol Sci. 2021. PMID: 33799890 Free PMC article. Review.
-
Overview of the Nucleic-Acid Binding Properties of the HIV-1 Nucleocapsid Protein in Its Different Maturation States.Viruses. 2020 Sep 29;12(10):1109. doi: 10.3390/v12101109. Viruses. 2020. PMID: 33003650 Free PMC article. Review.
Cited by
-
Computational identification and analysis of CNP0269688 as a natural product inhibitor disrupting the interaction between the HIV matrix domain and tRNA.Front Chem. 2024 Sep 2;12:1450339. doi: 10.3389/fchem.2024.1450339. eCollection 2024. Front Chem. 2024. PMID: 39286001 Free PMC article.
-
Viral Oncogenesis: Synergistic Role of Genome Integration and Persistence.Viruses. 2024 Dec 23;16(12):1965. doi: 10.3390/v16121965. Viruses. 2024. PMID: 39772271 Free PMC article. Review.
-
Understanding Post-Translational Modifications in Porcine Reproductive and Respiratory Syndrome Virus Infection.Vet Sci. 2024 Dec 16;11(12):654. doi: 10.3390/vetsci11120654. Vet Sci. 2024. PMID: 39728994 Free PMC article. Review.
-
Mechanism-guided engineering of a minimal biological particle for genome editing.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2413519121. doi: 10.1073/pnas.2413519121. Epub 2024 Dec 30. Proc Natl Acad Sci U S A. 2025. PMID: 39793042 Free PMC article.
References
-
- Bharat TAM, Castillo Menendez LR, Hagen WJH, Lux V, Igonet S, Schorb M, Schur FKM, Kräusslich H-G, Briggs JAG. Cryo-electron microscopy of tubular arrays of HIV-1 gag resolves structures essential for immature virus assembly. Proc Natl Acad Sci U S A. 2014;111:8233–8. doi: 10.1073/pnas.1401455111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

