An IL-10/DEL-1 axis supports granulopoiesis and survival from sepsis in early life
- PMID: 38263289
- PMCID: PMC10805706
- DOI: 10.1038/s41467-023-44178-y
An IL-10/DEL-1 axis supports granulopoiesis and survival from sepsis in early life
Abstract
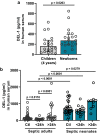
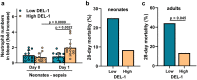
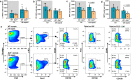
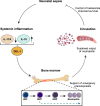
The limited reserves of neutrophils are implicated in the susceptibility to infection in neonates, however the regulation of neutrophil kinetics in infections in early life remains poorly understood. Here we show that the developmental endothelial locus (DEL-1) is elevated in neonates and is critical for survival from neonatal polymicrobial sepsis, by supporting emergency granulopoiesis. Septic DEL-1 deficient neonate mice display low numbers of myeloid-biased multipotent and granulocyte-macrophage progenitors in the bone marrow, resulting in neutropenia, exaggerated bacteremia, and increased mortality; defects that are rescued by DEL-1 administration. A high IL-10/IL-17A ratio, observed in newborn sepsis, sustains tissue DEL-1 expression, as IL-10 upregulates while IL-17 downregulates DEL-1. Consistently, serum DEL-1 and blood neutrophils are elevated in septic adult and neonate patients with high serum IL-10/IL-17A ratio, and mortality is lower in septic patients with high serum DEL-1. Therefore, IL-10/DEL-1 axis supports emergency granulopoiesis, prevents neutropenia and promotes sepsis survival in early life.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



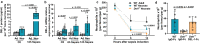


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

