Human whole-exome genotype data for Alzheimer's disease
- PMID: 38263370
- PMCID: PMC10805795
- DOI: 10.1038/s41467-024-44781-7
Human whole-exome genotype data for Alzheimer's disease
Abstract
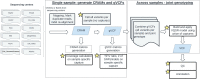
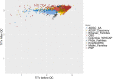
The heterogeneity of the whole-exome sequencing (WES) data generation methods present a challenge to a joint analysis. Here we present a bioinformatics strategy for joint-calling 20,504 WES samples collected across nine studies and sequenced using ten capture kits in fourteen sequencing centers in the Alzheimer's Disease Sequencing Project. The joint-genotype called variant-called format (VCF) file contains only positions within the union of capture kits. The VCF was then processed specifically to account for the batch effects arising from the use of different capture kits from different studies. We identified 8.2 million autosomal variants. 96.82% of the variants are high-quality, and are located in 28,579 Ensembl transcripts. 41% of the variants are intronic and 1.8% of the variants are with CADD > 30, indicating they are of high predicted pathogenicity. Here we show our new strategy can generate high-quality data from processing these diversely generated WES samples. The improved ability to combine data sequenced in different batches benefits the whole genomics research community.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

