Single-cell transcriptomics reveals that glial cells integrate homeostatic and circadian processes to drive sleep-wake cycles
- PMID: 38263460
- PMCID: PMC10849968
- DOI: 10.1038/s41593-023-01549-4
Single-cell transcriptomics reveals that glial cells integrate homeostatic and circadian processes to drive sleep-wake cycles
Abstract
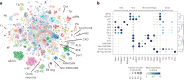
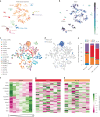
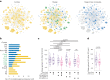
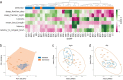
The sleep-wake cycle is determined by circadian and sleep homeostatic processes. However, the molecular impact of these processes and their interaction in different brain cell populations are unknown. To fill this gap, we profiled the single-cell transcriptome of adult Drosophila brains across the sleep-wake cycle and four circadian times. We show cell type-specific transcriptomic changes, with glia displaying the largest variation. Glia are also among the few cell types whose gene expression correlates with both sleep homeostat and circadian clock. The sleep-wake cycle and sleep drive level affect the expression of clock gene regulators in glia, and disrupting clock genes specifically in glia impairs homeostatic sleep rebound after sleep deprivation. These findings provide a comprehensive view of the effects of sleep homeostatic and circadian processes on distinct cell types in an entire animal brain and reveal glia as an interaction site of these two processes to determine sleep-wake dynamics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





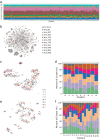
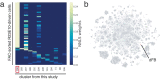

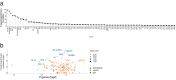

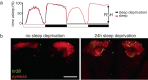

Similar articles
-
The sleep-wake distribution contributes to the peripheral rhythms in PERIOD-2.Elife. 2021 Dec 13;10:e69773. doi: 10.7554/eLife.69773. Elife. 2021. PMID: 34895464 Free PMC article.
-
Sleep-wake-driven and circadian contributions to daily rhythms in gene expression and chromatin accessibility in the murine cortex.Proc Natl Acad Sci U S A. 2019 Dec 17;116(51):25773-25783. doi: 10.1073/pnas.1910590116. Epub 2019 Nov 27. Proc Natl Acad Sci U S A. 2019. PMID: 31776259 Free PMC article.
-
Stress response genes protect against lethal effects of sleep deprivation in Drosophila.Nature. 2002 May 16;417(6886):287-91. doi: 10.1038/417287a. Nature. 2002. PMID: 12015603
-
Circadian clock genes and sleep homeostasis.Eur J Neurosci. 2009 May;29(9):1820-9. doi: 10.1111/j.1460-9568.2009.06723.x. Epub 2009 Apr 28. Eur J Neurosci. 2009. PMID: 19473235 Review.
-
Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus.Brain Res Brain Res Rev. 2005 Nov;49(3):429-54. doi: 10.1016/j.brainresrev.2005.01.005. Brain Res Brain Res Rev. 2005. PMID: 16269313 Review.
Cited by
-
Insect circadian plasticity as a proposed target for the expression of parasite extended phenotypes.NPJ Biol Timing Sleep. 2025;2(1):29. doi: 10.1038/s44323-025-00046-0. Epub 2025 Aug 1. NPJ Biol Timing Sleep. 2025. PMID: 40755487 Free PMC article. Review.
-
Glia: the cellular glue that binds circadian rhythms and sleep.Sleep. 2025 Mar 11;48(3):zsae314. doi: 10.1093/sleep/zsae314. Sleep. 2025. PMID: 39812780 Free PMC article. Review.
-
Stress-Induced Sleep Dysregulation: The Roles of Astrocytes and Microglia in Neurodegenerative and Psychiatric Disorders.Biomedicines. 2025 May 6;13(5):1121. doi: 10.3390/biomedicines13051121. Biomedicines. 2025. PMID: 40426947 Free PMC article. Review.
-
Microbiome drives age-dependent shifts in brain transcriptomic programs at the single-cell level in Drosophila.NPJ Biofilms Microbiomes. 2025 Aug 12;11(1):162. doi: 10.1038/s41522-025-00781-z. NPJ Biofilms Microbiomes. 2025. PMID: 40796784 Free PMC article.
-
Dynamics of glia and neurons regulate homeostatic rest, sleep and feeding behavior in Drosophila.Nat Neurosci. 2025 Jun;28(6):1226-1240. doi: 10.1038/s41593-025-01942-1. Epub 2025 Apr 21. Nat Neurosci. 2025. PMID: 40259071 Free PMC article.
References
-
- Borbély AA. A two process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. - PubMed
-
- Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020;21:67–84. - PubMed
-
- Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018;19:453–469. - PubMed
-
- Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. - PubMed
MeSH terms
Grants and funding
- 758580/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- G052922N/Fonds Wetenschappelijk Onderzoek (Research Foundation Flanders)
- 11D8820N/Fonds Wetenschappelijk Onderzoek (Research Foundation Flanders)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

