Functionally-selective inhibition of threshold sodium currents and excitability in dorsal root ganglion neurons by cannabinol
- PMID: 38263462
- PMCID: PMC10805714
- DOI: 10.1038/s42003-024-05781-x
Functionally-selective inhibition of threshold sodium currents and excitability in dorsal root ganglion neurons by cannabinol
Abstract
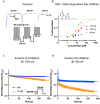
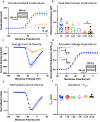
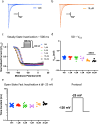
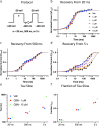
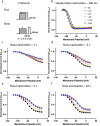
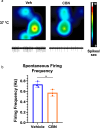
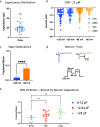
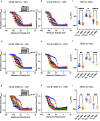
Cannabinol (CBN), an incompletely understood metabolite for ∆9-tetrahydrocannabinol, has been suggested as an analgesic. CBN interacts with endocannabinoid (CB) receptors, but is also reported to interact with non-CB targets, including various ion channels. We assessed CBN effects on voltage-dependent sodium (Nav) channels expressed heterologously and in native dorsal root ganglion (DRG) neurons. Our results indicate that CBN is a functionally-selective, but structurally-non-selective Nav current inhibitor. CBN's main effect is on slow inactivation. CBN slows recovery from slow-inactivated states, and hyperpolarizes steady-state inactivation, as channels enter deeper and slower inactivated states. Multielectrode array recordings indicate that CBN attenuates DRG neuron excitability. Voltage- and current-clamp analysis of freshly isolated DRG neurons via our automated patch-clamp platform confirmed these findings. The inhibitory effects of CBN on Nav currents and on DRG neuron excitability add a new dimension to its actions and suggest that this cannabinoid may be useful for neuropathic pain.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Inhibition of sodium conductance by cannabigerol contributes to a reduction of dorsal root ganglion neuron excitability.Br J Pharmacol. 2022 Aug;179(15):4010-4030. doi: 10.1111/bph.15833. Epub 2022 Apr 22. Br J Pharmacol. 2022. PMID: 35297036
-
A novel selective and orally bioavailable Nav 1.8 channel blocker, PF-01247324, attenuates nociception and sensory neuron excitability.Br J Pharmacol. 2015 May;172(10):2654-70. doi: 10.1111/bph.13092. Epub 2015 Apr 10. Br J Pharmacol. 2015. PMID: 25625641 Free PMC article.
-
A novel isoquinoline alkaloid HJ-69 isolated from Zanthoxylum bungeanum attenuates inflammatory pain by inhibiting voltage-gated sodium and potassium channels.J Ethnopharmacol. 2024 Aug 10;330:118218. doi: 10.1016/j.jep.2024.118218. Epub 2024 Apr 25. J Ethnopharmacol. 2024. PMID: 38677570
-
Nav1.7-A1632G Mutation from a Family with Inherited Erythromelalgia: Enhanced Firing of Dorsal Root Ganglia Neurons Evoked by Thermal Stimuli.J Neurosci. 2016 Jul 13;36(28):7511-22. doi: 10.1523/JNEUROSCI.0462-16.2016. J Neurosci. 2016. PMID: 27413160 Free PMC article.
-
Tetrodotoxin-Sensitive Sodium Channels Mediate Action Potential Firing and Excitability in Menthol-Sensitive Vglut3-Lineage Sensory Neurons.J Neurosci. 2019 Sep 4;39(36):7086-7101. doi: 10.1523/JNEUROSCI.2817-18.2019. Epub 2019 Jul 12. J Neurosci. 2019. PMID: 31300524 Free PMC article.
Cited by
-
Distinct Interactions of Cannabinol and Its Cytochrome P450-Generated Metabolites with Receptors and Sensory Neurons.J Med Chem. 2025 Jul 10;68(13):13935-13953. doi: 10.1021/acs.jmedchem.5c00938. Epub 2025 Jun 26. J Med Chem. 2025. PMID: 40568800 Free PMC article.
-
Nav1.8, an analgesic target for nonpsychotomimetic phytocannabinoids.Proc Natl Acad Sci U S A. 2025 Jan 28;122(4):e2416886122. doi: 10.1073/pnas.2416886122. Epub 2025 Jan 21. Proc Natl Acad Sci U S A. 2025. PMID: 39835903 Free PMC article.
-
In vitro inhibition of voltage-dependent sodium currents by the antifungal drug amorolfine.J Biol Chem. 2025 Apr;301(4):108407. doi: 10.1016/j.jbc.2025.108407. Epub 2025 Mar 14. J Biol Chem. 2025. PMID: 40090585 Free PMC article.
References
-
- Jan, T. R., Farraj, A. K., Harkema, J. R. & Kaminski, N. E. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol. Appl. Pharmacol.188, 24–35 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

