Minimally invasive surgery for clinical T4 non-small-cell lung cancer: national trends and outcomes
- PMID: 38263602
- PMCID: PMC11007735
- DOI: 10.1093/ejcts/ezae009
Minimally invasive surgery for clinical T4 non-small-cell lung cancer: national trends and outcomes
Erratum in
-
Corrigendum to: Minimally invasive surgery for clinical T4 non-small-cell lung cancer: national trends and outcomes; Is underutilization of adjuvant therapy in resected non-small-cell lung cancer associated with socioeconomic disparities?; and Sublobar resection is associated with less lymph nodes examined and lower delivery of adjuvant therapy in patients with 1.5- to 2.0-cm clinical IA2 non-small-cell lung cancer: a retrospective cohort study.Eur J Cardiothorac Surg. 2024 May 3;65(5):ezae203. doi: 10.1093/ejcts/ezae203. Eur J Cardiothorac Surg. 2024. PMID: 38783681 Free PMC article. No abstract available.
Abstract
Objectives: Recent randomized data support the perioperative benefits of minimally invasive surgery (MIS) for non-small-cell lung cancer (NSCLC). Its utility for cT4 tumours remains understudied. We, therefore, sought to analyse national trends and outcomes of minimally invasive resections for cT4 cancers.
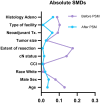
Methods: Using the 2010-2019 National Cancer Database, we identified patients with cT4N0-1 NSCLC. Patients were stratified by surgical approach. Multivariable logistic analysis was used to identify factors associated with use of a minimally invasive approach. Groups were matched using propensity score analysis to evaluate perioperative and survival end points.
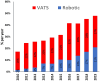
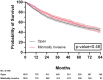
Results: The study identified 3715 patients, among whom 64.1% (n = 2381) underwent open resection and 35.9% (n = 1334) minimally invasive resection [robotic-assisted in 31.5% (n = 420); and video-assisted in 68.5% (n = 914)]. Increased MIS use was noted among patients with higher income [≥$40 227, odds ratio (OR) 1.24; 95% confidence interval (CI) 1.01-1.51] and those treated at academic hospitals (OR 1.25; 95% CI 1.07-1.45). Clinically node-positive patients (OR 0.68; 95% CI 0.55-0.83) and those who underwent neoadjuvant therapy (OR 0.78; 95% CI 0.65-0.93) were less likely to have minimally invasive resection. In matched groups, patients undergoing MIS had a shorter median length of stay (5 vs 6 days, P < 0.001) and no significant differences between 30-day readmissions or 30/90-day mortality. MIS did not compromise overall survival (log-rank P = 0.487).
Conclusions: Nationally, the use of minimally invasive approaches for patients with cT4N0-1M0 NSCLC has increased substantially. In these patients, MIS is safe and does not compromise perioperative outcomes or survival.
Keywords: Lung cancer; Minimally invasive surgery; Robotic-assisted thoracoscopic surgery; Thoracotomy; Video-assisted thoracoscopic surgery.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery. All rights reserved.
Figures

Comment in
-
The role of minimally invasive surgery on cT4 tumours: still many unanswered question.Eur J Cardiothorac Surg. 2024 Mar 1;65(3):ezae049. doi: 10.1093/ejcts/ezae049. Eur J Cardiothorac Surg. 2024. PMID: 38341663 No abstract available.
References
-
- Potter AL, Spasojevic A, Raman V, Hurd JC, Senthil P, Mathey-Andrews C et al. The increasing adoption of minimally invasive lobectomy in the United States. Ann Thorac Surg 2023;116:222–9. - PubMed
-
- Paul S, Sedrakyan A, Chiu Y, Nasar A, Port JL, Lee PC et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813–7. - PubMed
-
- Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836–44. - PubMed
-
- Lim EKS, Batchelor TJP, Dunning J, Shackcloth M, Anikin V, Naidu B et al. Video-assisted thoracoscopic versus open lobectomy in patients with early-stage lung cancer: one-year results from a randomized controlled trial (VIOLET). JCO 2021;39:8504.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

