Unlocking antitumor immunity with adenosine receptor blockers
- PMID: 38263981
- PMCID: PMC10804392
- DOI: 10.20517/cdr.2023.63
Unlocking antitumor immunity with adenosine receptor blockers
Abstract
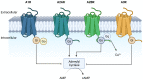
Tumors survive by creating a tumor microenvironment (TME) that suppresses antitumor immunity. The TME suppresses the immune system by limiting antigen presentation, inhibiting lymphocyte and natural killer (NK) cell activation, and facilitating T cell exhaustion. Checkpoint inhibitors like anti-PD-1 and anti-CTLA4 are immunostimulatory antibodies, and their blockade extends the survival of some but not all cancer patients. Extracellular adenosine triphosphate (ATP) is abundant in inflamed tumors, and its metabolite, adenosine (ADO), is a driver of immunosuppression mediated by adenosine A2A receptors (A2AR) and adenosine A2B receptors (A2BR) found on tumor-associated lymphoid and myeloid cells. This review will focus on adenosine as a key checkpoint inhibitor-like immunosuppressive player in the TME and how reducing adenosine production or blocking A2AR and A2BR enhances antitumor immunity.
Keywords: Immunotherapy; adenosine; adenosine A2A receptors (A2AR); adenosine A2B receptors (A2BR); adenosine receptors; immune cells; tumor cells; tumor microenvironment.
© The Author(s) 2023.
Conflict of interest statement
Drs. Dimastromatteo and Linden are members of Adovate, a for-profit company dedicated to improving patient health using a new generation of adenosine analogs.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
