In Silico Analysis Determining the Binding Interactions of NAD(P)H: Quinone Oxidoreductase 1 and Resveratrol via Docking and Molecular Dynamic Simulations
- PMID: 38264080
- PMCID: PMC10805530
- DOI: 10.26650/eurjbiol.2023.1352396
In Silico Analysis Determining the Binding Interactions of NAD(P)H: Quinone Oxidoreductase 1 and Resveratrol via Docking and Molecular Dynamic Simulations
Abstract
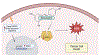
Objective: NAD(P)H: Quinone oxidoreductase1 (NQO1) plays a crucial role in cellular defense against oxidative stress. Overexpression of NQO1 is linked to various cancer pathways. Despite its potential, the actual mechanisms to inhibit NQO1 and increase the efficacy of standard therapeutic options are not yet established. Resveratrol is an anti-cancer polyphenol found in dietary products and red wine. The objective of this investigation is to employ in silico methods to explore how resveratrol interacts with NQO1.
Materials and methods: Docking analysis of resveratrol against NQO1 was performed using Glide. The most efficiently docked complex was characterized and analyzed by measuring intermolecular (IM) hydrogen (H)-bonds and binding energy values, additional hydrophobic, and electrostatic interactions. IM interaction between complexed protein and compound was demonstrated using LigPlot+ and the Schrödinger ligand interaction module. Molecular dynamics tools were employed to examine the physical movement of molecules to evaluate how macromolecular structures relate to their functions.
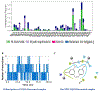
Results: The results of this investigation depicted a strong affinity of resveratrol against NQO1 followed by MD simulations (NQO1-resveratrol complex-binding energy: -2.847kcal/mol). Resveratrol's robust binding affinity through docking and molecular dynamic simulations highlights a significant change around 90 ns. The H-bonds number was inversely linked with the resveratrol-NQO1 complex stability. The NQO1-Resveratrol complex displayed dynamic motion, as revealed by porcupine projections, indicating alterations in its movement and flexibility.
Conclusion: The present in silico analysis suggests a possible alteration in resveratrol's orientation in the protein binding pocket. The findings encourage further investigation, including validation using in vitro and in vivo assays.
Keywords: In silico Analysis; Molecular Dynamic Simulation; NQO1; Oxidative Stress; Resveratrol.
Conflict of interest statement
Conflict of Interest: Authors declared no conflict of interest.
Figures
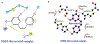


References
-
- Preethi S, Arthiga K, Patil AB, Spandana A, Jain V. Review on NAD(P)H dehydrogenase quinone 1 (NQO1) pathway. Mol Biol Rep. 2022;49(9):8907–8924 - PubMed
-
- Beaver SK, Mesa-Torres N, Pey AL, Timson DJ. NQO1: A target for treating cancer and neurological diseases, and a model to understand loss of function disease mechanisms. Biochim Biophys Acta Proteins Proteom. 2019;1867(7-8):663–676. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
