Placental cytochrome P450 methylomes in infants exposed to prenatal opioids: exploring the effects of neonatal opioid withdrawal syndrome on health horizons
- PMID: 38264209
- PMCID: PMC10805101
- DOI: 10.3389/fgene.2023.1292148
Placental cytochrome P450 methylomes in infants exposed to prenatal opioids: exploring the effects of neonatal opioid withdrawal syndrome on health horizons
Abstract
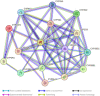
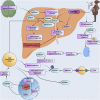
Background: Neonatal opioid withdrawal syndrome (NOWS), arises due to increased opioid use during pregnancy. Cytochrome P450 (CYP) enzymes play a pivotal role in metabolizing a wide range of substances in the human body, including opioids, other drugs, toxins, and endogenous compounds. The association between CYP gene methylation and opioid effects is unexplored and it could offer promising insights. Objective: To investigate the impact of prenatal opioid exposure on disrupted CYPs in infants and their anticipated long-term clinical implications. Study Design: DNA methylation levels of CYP genes were analyzed in a cohort of 96 placental tissues using Illumina Infinium MethylationEPIC (850 k) BeadChips. This involved three groups of placental tissues: 32 from mothers with infants exposed to opioids prenatally requiring pharmacologic treatment for NOWS, 32 from mothers with prenatally opioid-exposed infants not needing NOWS treatment, and 32 from unexposed control mothers. Results: The study identified 20 significantly differentially methylated CpG sites associated with 17 distinct CYP genes, with 14 CpGs showing reduced methylation across 14 genes (CYP19A1, CYP1A2, CYP4V2, CYP1B1, CYP24A1, CYP26B1, CYP26C1, CYP2C18, CYP2C9, CYP2U1, CYP39A1, CYP2R1, CYP4Z1, CYP2D7P1 and), while 8 exhibited hypermethylation (CYP51A1, CYP26B1, CYP2R1, CYP2U1, CYP4X1, CYP1A2, CYP2W1, and CYP4V2). Genes such as CYP1A2, CYP26B1, CYP2R1, CYP2U1, and CYP4V2 exhibited both increased and decreased methylation. These genes are crucial for metabolizing eicosanoids, fatty acids, drugs, and diverse substances. Conclusion: The study identified profound methylation changes in multiple CYP genes in the placental tissues relevant to NOWS. This suggests that disruption of DNA methylation patterns in CYP transcripts might play a role in NOWS and may serve as valuable biomarkers, suggesting a future pathway for personalized treatment. Further research is needed to confirm these findings and explore their potential for diagnosis and treatment.
Keywords: CYP1A2; CYP1B1; CYP24A1; CYP4V2; biomarker; cytochromes; neonatal opioid withdrawal syndrome CYP19A1; opioid use.
Copyright © 2024 Radhakrishna, Sadhasivam, Radhakrishnan, Forray, Muvvala, Metpally, Patel, Rawal, Vishweswaraiah, Bahado-Singh and Nath.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Prenatal opioid exposure significantly impacts placental protein kinase C (PKC) and drug transporters, leading to drug resistance and neonatal opioid withdrawal syndrome.Front Neurosci. 2024 Aug 19;18:1442915. doi: 10.3389/fnins.2024.1442915. eCollection 2024. Front Neurosci. 2024. PMID: 39238930 Free PMC article.
-
Prenatal opioid exposure alters pain perception and increases long-term health risks in infants with neonatal opioid withdrawal syndrome.Front Pain Res (Lausanne). 2025 Apr 17;6:1497801. doi: 10.3389/fpain.2025.1497801. eCollection 2025. Front Pain Res (Lausanne). 2025. PMID: 40313396 Free PMC article.
-
Placental microRNA methylome signatures may serve as biomarkers and therapeutic targets for prenatally opioid-exposed infants with neonatal opioid withdrawal syndrome.Front Genet. 2023 Jun 15;14:1215472. doi: 10.3389/fgene.2023.1215472. eCollection 2023. Front Genet. 2023. PMID: 37434949 Free PMC article.
-
Reconceptualizing non-pharmacologic approaches to Neonatal Abstinence Syndrome (NAS) and Neonatal Opioid Withdrawal Syndrome (NOWS): A theoretical and evidence-based approach. Part II: The clinical application of nonpharmacologic care for NAS/NOWS.Neurotoxicol Teratol. 2021 Nov-Dec;88:107032. doi: 10.1016/j.ntt.2021.107032. Epub 2021 Sep 29. Neurotoxicol Teratol. 2021. PMID: 34600100 Review.
-
Reconceptualizing non-pharmacologic approaches to Neonatal Abstinence Syndrome (NAS) and Neonatal Opioid Withdrawal Syndrome (NOWS): A theoretical and evidence-based approach.Neurotoxicol Teratol. 2021 Nov-Dec;88:107020. doi: 10.1016/j.ntt.2021.107020. Epub 2021 Aug 19. Neurotoxicol Teratol. 2021. PMID: 34419619 Free PMC article. Review.
Cited by
-
Research progress of DNA methylation on the regulation of substance use disorders and the mechanisms.Front Cell Neurosci. 2025 Mar 31;19:1566001. doi: 10.3389/fncel.2025.1566001. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40230379 Free PMC article. Review.
-
Prenatal opioid exposure significantly impacts placental protein kinase C (PKC) and drug transporters, leading to drug resistance and neonatal opioid withdrawal syndrome.Front Neurosci. 2024 Aug 19;18:1442915. doi: 10.3389/fnins.2024.1442915. eCollection 2024. Front Neurosci. 2024. PMID: 39238930 Free PMC article.
-
Prenatal opioid exposure alters pain perception and increases long-term health risks in infants with neonatal opioid withdrawal syndrome.Front Pain Res (Lausanne). 2025 Apr 17;6:1497801. doi: 10.3389/fpain.2025.1497801. eCollection 2025. Front Pain Res (Lausanne). 2025. PMID: 40313396 Free PMC article.
References
-
- Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N. J., et al. (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. Berl. 210 (5-6), 343–352. 10.1007/s00429-005-0025-5 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

