Bronchodilator Responsiveness Measured by Spirometry and Impulse Oscillometry in Patients with Asthma After Short Acting Antimuscarinic and/or Beta-2-Agonists Inhalation
- PMID: 38264293
- PMCID: PMC10804873
- DOI: 10.2147/JAA.S442217
Bronchodilator Responsiveness Measured by Spirometry and Impulse Oscillometry in Patients with Asthma After Short Acting Antimuscarinic and/or Beta-2-Agonists Inhalation
Abstract
Background: Bronchodilator responsiveness (BDR) in asthma involves both the central and peripheral airways but is primarily relieved with beta-2-agonists and evaluated by spirometry. To date, antimuscarinics can be added as a reliever medication in more severe asthma. We hypothesize that combining both short-acting beta-2 agonist (SABA) and short-acting muscarinic antagonist (SAMA) could also improve the responsiveness in mild-moderate asthma. Therefore, we aimed to compare the direct effects of inhaling SABA alone, SAMA alone or combining both SABA and SAMA on the central and peripheral airways in asthma.
Methods: Twenty-three patients with mild-moderate BDR in asthma performed dynamic spirometry and impulse oscillometry before (baseline) and multiple timepoints within an hour after inhalation of SABA (salbutamol), SAMA (ipratropium bromide), or both SABA and SAMA at three different visits.
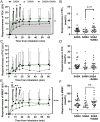
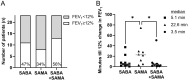
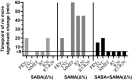
Results: The use of SAMA alone did not show any improvement compared to the use of SABA alone. Inhalation of SABA+SAMA, however, averaged either similar or better BDR than SABA alone in FEV1, MMEF, FVC, R5, R20 and R5-R20. Inhaling SABA+SAMA reached a stable BDR in more patients within 0-10 minutes and also reached the FEV1 (Δ%)>12% faster (3.5 minutes) than inhaling SABA alone (5.1 minutes). Inhaling SABA+SAMA was significantly better than SAMA alone in FEV1 (p = 0.015), MMEF (p = 0.0059) and R20 (p = 0.0049). Using these three variables highlighted a subgroup (30%, including more males) of patients that were more responsive to inhaling SABA+SAMA than SABA alone.
Conclusion: Overall, combining SAMA with SABA was faster and more consistent at increasing the lung function than SABA alone or SAMA alone, and the additive effect was best captured by incorporating peripheral-related variables. Therefore, SAMA should be considered as an add-on reliever for mild-moderate patients with BDR in asthma.
Keywords: BDR; SABA; SAMA; central airway; mild-moderate asthma; peripheral airways.
© 2024 van der Burg et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Assessment of bronchodilator responsiveness to salbutamol or ipratropium using different criteria in treatment-naïve patients with asthma and COPD.Eur Clin Respir J. 2024 Mar 21;11(1):2328434. doi: 10.1080/20018525.2024.2328434. eCollection 2024. Eur Clin Respir J. 2024. PMID: 38529514 Free PMC article.
-
Assessment of spirometry and impulse oscillometry in relation to asthma control.Lung. 2015 Feb;193(1):47-51. doi: 10.1007/s00408-014-9674-6. Epub 2014 Dec 17. Lung. 2015. PMID: 25516285
-
As-needed ICS-LABA in Mild Asthma: What Does the Evidence Say?Drugs. 2019 Nov;79(16):1729-1737. doi: 10.1007/s40265-019-01202-0. Drugs. 2019. PMID: 31584145 Review.
-
The management of mild asthma.Eur Respir J. 2021 Apr 8;57(4):2003051. doi: 10.1183/13993003.03051-2020. Print 2021 Apr. Eur Respir J. 2021. PMID: 33093120 Review.
References
-
- Asthma. GIf. Global Strategy for Asthma Management and Prevention, 2022; 2022. https://ginasthma.org/gina-reports/. Accessed December 4, 2024.
LinkOut - more resources
Full Text Sources

