Revving the engine: PKB/AKT as a key regulator of cellular glucose metabolism
- PMID: 38264327
- PMCID: PMC10804622
- DOI: 10.3389/fphys.2023.1320964
Revving the engine: PKB/AKT as a key regulator of cellular glucose metabolism
Abstract
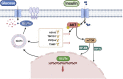
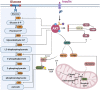
Glucose metabolism is of critical importance for cell growth and proliferation, the disorders of which have been widely implicated in cancer progression. Glucose uptake is achieved differently by normal cells and cancer cells. Even in an aerobic environment, cancer cells tend to undergo metabolism through glycolysis rather than the oxidative phosphorylation pathway. Disordered metabolic syndrome is characterized by elevated levels of metabolites that can cause changes in the tumor microenvironment, thereby promoting tumor recurrence and metastasis. The activation of glycolysis-related proteins and transcription factors is involved in the regulation of cellular glucose metabolism. Changes in glucose metabolism activity are closely related to activation of protein kinase B (PKB/AKT). This review discusses recent findings on the regulation of glucose metabolism by AKT in tumors. Furthermore, the review summarizes the potential importance of AKT in the regulation of each process throughout glucose metabolism to provide a theoretical basis for AKT as a target for cancers.
Keywords: AKT; cancer; glucose glycolysis; glucose uptake; tricarboxylic acid cycle.
Copyright © 2024 Li, Hu, Cai, Liu, Luo and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Computational modeling of the metabolic States regulated by the kinase akt.Front Physiol. 2012 Nov 21;3:418. doi: 10.3389/fphys.2012.00418. eCollection 2012. Front Physiol. 2012. PMID: 23181020 Free PMC article.
-
Metabolic phenotype of bladder cancer.Cancer Treat Rev. 2016 Apr;45:46-57. doi: 10.1016/j.ctrv.2016.03.005. Epub 2016 Mar 8. Cancer Treat Rev. 2016. PMID: 26975021 Review.
-
The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism.Cancer Lett. 2015 Jan 28;356(2 Pt A):156-64. doi: 10.1016/j.canlet.2014.04.001. Epub 2014 Apr 13. Cancer Lett. 2015. PMID: 24732809 Free PMC article. Review.
-
Halofuginone inhibits colorectal cancer growth through suppression of Akt/mTORC1 signaling and glucose metabolism.Oncotarget. 2015 Sep 15;6(27):24148-62. doi: 10.18632/oncotarget.4376. Oncotarget. 2015. PMID: 26160839 Free PMC article.
-
Proliferating tumor cells mimick glucose metabolism of mature human erythrocytes.Cell Cycle. 2019 Jun;18(12):1316-1334. doi: 10.1080/15384101.2019.1618125. Epub 2019 Jun 3. Cell Cycle. 2019. PMID: 31154896 Free PMC article. Review.
Cited by
-
Comparative analysis of PI3K-AKT and MEK-ERK1/2 signaling-driven molecular changes in granulosa cells.Reproduction. 2025 Jan 21;169(2):e240317. doi: 10.1530/REP-24-0317. Print 2025 Feb 1. Reproduction. 2025. PMID: 39665647 Free PMC article.
-
Combined Stromal Vascular Fraction and Fractional CO2 Laser Therapy for Hypertrophic Scar Treatment: A Systematic Review and Meta-Analysis.Aesthetic Plast Surg. 2025 Feb;49(3):885-896. doi: 10.1007/s00266-024-04359-6. Epub 2024 Sep 27. Aesthetic Plast Surg. 2025. PMID: 39333369
-
Inhibition of serotonin-Htr2b signaling in skeletal muscle mitigates obesity-induced insulin resistance.Exp Mol Med. 2025 Jun;57(6):1177-1188. doi: 10.1038/s12276-025-01460-x. Epub 2025 Jun 2. Exp Mol Med. 2025. PMID: 40451926 Free PMC article.
-
Enhanced ECCD36 signaling promotes skeletal muscle insulin resistance in female mice.Am J Physiol Endocrinol Metab. 2024 Oct 1;327(4):E533-E543. doi: 10.1152/ajpendo.00246.2024. Epub 2024 Aug 28. Am J Physiol Endocrinol Metab. 2024. PMID: 39196801
-
Enfortumab Vedotin-Induced Diabetic Ketoacidosis and Acute Tubulointerstitial Nephritis Requiring Intensive Care in the Treatment of Advanced Urothelial Carcinoma: A Case Report.Case Rep Oncol. 2025 Apr 29;18(1):667-674. doi: 10.1159/000545957. eCollection 2025 Jan-Dec. Case Rep Oncol. 2025. PMID: 40487556 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

