Maternal prenatal, with or without postpartum, vitamin D3 supplementation does not improve maternal iron status at delivery or infant iron status at 6 months of age: secondary analysis of a randomised controlled trial
- PMID: 38264359
- PMCID: PMC10800272
- DOI: 10.1136/bmjnph-2023-000758
Maternal prenatal, with or without postpartum, vitamin D3 supplementation does not improve maternal iron status at delivery or infant iron status at 6 months of age: secondary analysis of a randomised controlled trial
Abstract
Background: Vitamin D may modify iron status through regulation of hepcidin and inflammatory pathways. This study aimed to investigate effects of maternal vitamin D supplementation on iron status in pregnancy and early infancy.
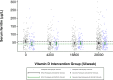
Methods: In a trial in Dhaka, Bangladesh, women (n=1300) were randomised to one of five vitamin D3 regimens from 17 to 24 weeks' gestation until 26 weeks postpartum (prenatal; postpartum doses): 0;0, 4200;0, 16 800;0, 28 000;0 or 28 000;28 000 IU/week. All participants received standard iron-folic acid supplementation. In this secondary analysis (n=998), we examined effects of prenatal;postpartum vitamin D on serum ferritin and other biomarkers of maternal iron status (transferrin saturation, total iron binding capacity, soluble transferrin receptor and hepcidin) at delivery, and infant ferritin and haemoglobin at 6 months of age. Using linear regression, we estimated per cent mean differences between each intervention group and placebo with 95% CIs, with and without adjustment for baseline ferritin or inflammatory biomarkers (C reactive protein and α-1-acid glycoprotein (AGP)).
Results: At delivery, ferritin concentrations were similar between each intervention group and placebo in unadjusted (n=998) and baseline ferritin-adjusted analyses (n=992; p>0.05). Compared with placebo, AGP was lower in each intervention group (per cent difference (95% CI) = -11% (-21 to -1.0), -14% (-23 to -3.5) and -11% (-19 to -2.0) in the 4200 IU/week, 16 800 IU/week and 28 000 IU/week groups, respectively; n=779). In the subgroup of women with baseline 25-hydroxyvitamin D < 30 nmol/L, ferritin was lower in each intervention group versus placebo (-23% (-37 to -5.0), -20% (-35 to -1.9) and -20% (-33 to -4.1) in the 4200 IU/week, 16 800 IU/week and 28 000 IU/week groups, respectively; n=645); effects were slightly attenuated after adjustment for inflammation (n=510). There were no effects of vitamin D on other iron biomarkers among women at delivery or infants aged 6 months.
Conclusion: These findings do not support improvement of iron status by vitamin D. The effect of prenatal vitamin D supplementation on ferritin may reflect an anti-inflammatory mechanism.
Keywords: Nutrient deficiencies.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared
Figures
References
-
- Stevens GA, Finucane MM, De-Regil LM, et al. . Global, regional, and national trends in Haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health 2013;1:e16–25. 10.1016/S2214-109X(13)70001-9 - DOI - PMC - PubMed
-
- Stevens GA, Beal T, Mbuya MNN, et al. . Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys. Lancet Glob Health 2022;10:e1590–9. 10.1016/S2214-109X(22)00367-9 - DOI - PMC - PubMed
-
- World Health Organization . WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. World Health Organization, 2016. Available: https://www.who.int/publications/i/item/9789241549912 [accessed 4 Aug 2023]. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
