Meloidogyne enterolobii-induced Changes in Guava Root Exudates Are Associated With Root Rotting Caused by Neocosmospora falciformis
- PMID: 38264459
- PMCID: PMC10805520
- DOI: 10.2478/jofnem-2023-0055
Meloidogyne enterolobii-induced Changes in Guava Root Exudates Are Associated With Root Rotting Caused by Neocosmospora falciformis
Abstract
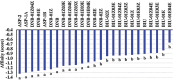
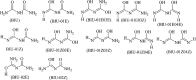
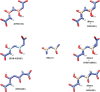
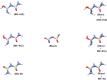
Despite the worldwide importance of disease complexes involving root-feeding nematodes and soilborne fungi, there have been few in-depth studies on how these organisms interact at the molecular level. Previous studies of guava decline have shown that root exudates from Meloidogyne enterolobii-parasitized guava plants (NP plants), but not from nematode-free plants (NF plants), enable the fungus Neocosmospora falciformis to rot guava roots, leading to plant death. To further characterize this interaction, NP and NF root exudates were lyophilized; extracted with distinct solvents; quantified regarding amino acids, soluble carbohydrates, sucrose, phenols, and alkaloids; and submitted to a bioassay to determine their ability to enable N. falciformis to rot the guava seedlings' roots. NP root exudates were richer than NF root exudates in amino acids, carbohydrates, and sucrose. Only the fractions NP-03 and NP-04 enabled fungal root rotting. NP-03 was then sequentially fractionated through chromatographic silica columns. At each step, the main fractions were reassessed in bioassay. The final fraction that enabled fungal root rotting was submitted to analysis using high performance liquid chromatography, nuclear magnetic resonance, mass spectrometry, energy-dispersive X-ray fluorescence, and computational calculations, leading to the identification of 1,5-dinitrobiuret as the predominant substance. In conclusion, parasitism by M. enterolobii causes an enrichment of guava root exudates that likely favors microorganisms capable of producing 1,5-dinitrobiuret in the rhizosphere. The accumulation of biuret, a known phytotoxic substance, possibly hampers root physiology and the innate immunity of guava to N. falciformis.
Keywords: 1,5-dinitrobiuret; Meloidogyne enterolobii; Neocosmospora falciformis; Psidium guajava; disease complex; guava; guava decline; nematode-fungus interaction; root exudate; root-knot nematode.
© 2023 Ricardo M. Souza et al., published by Sciendo.
Figures








References
-
- Abawi G. S., Chen J. Barker K. R., Pederson G. A., Windham G. L., Bartels J. M. Plant and nematode interactions. Agronomy monograph 36. Madison: American Society of Agronomy, Crop Science Society of America and Soil Science Society of America; 1998. Concomitant pathogen and pest interactions; pp. 135–158.
-
- Anonymous. Official methods of analysis. Arlington: Association of Official Analytical Chemists; 1996.
-
- Aprà E., Bylaska E. J., de Jong W. A, Govind N., Kowalski K., Straatsma T. P., Valiev M., van Dam H. J. J., Alexeev Y., Anchell J., Anisimov V., Aquino F. W., Atta-Fynn R., Autschbach J., Bauman N. P., Becca J. C., Bernholdt D. E., Bhaskaran-Nair K., Bogatko S., Borowski P., Boschen J., Brabec J., Bruner A., Cauët E., Chen Y., Chuev G. N., Cramer C. J., Daily J., Deegan M. J. O., Dunning T. H. Jr., Dupuis M., Dyall K. G., Fann G. I., Fischer S. A., Fonari A., Früchtl H., Gagliardi L., Garza J., Gawande N., Ghosh S., Glaesemann K., Götz A. W., Hammond J., Helms V., Hermes E. D., Hirao K., Hirata S., Jacquelin M., Jensen L., Johnson B. G., Jónsson H., Kendall R. A., Klemm M., Kobayashi R., Konkov V., Krishnamoorthy S., Krishnan M., Lin Z., Lins R. D., Littlefield R. J., Logsdail A. J., Lopata K., Ma W., Marenich A. V., Martin del Campo J., Mejia-Rodriguez D., Moore J. E., Mullin J. M., Nakajima T., Nascimento D. R., Nichols J. A., Nichols P. J., Nieplocha J., Otero-de-la-Roza A., Palmer B., Panyala A., Pirojsirikul T., Peng B., Peverati R., Pittner J., Pollack L., Richard R. M., Sadayappan P., Schatz G. C., Shelton W. A., Silverstein D. W., Smith D. M. A., Soares T. A., Song D., Swart M., Taylor H. L., Thomas G. S., Tipparaju V., Truhlar D. G., Tsemekhman K., Van Voorhis T., Vázquez-Mayagoitia A., Verma P., Villa O., Vishnu A., Vogiatzis K. D., Wang D., Weare J. H., Williamson M. J., Windus T. L., Woliński K., Wong A. T., Wu Q., Yang C., Yu Q., Zacharias M., Zhang Z., Zhao Y., Harrison R. J.. NWChem: past, present, and future. The Journal of Chemical Physics. 2020;152:184102. doi: 10.1063/5.0004997. - DOI - PubMed
-
- Back M. A., Haydock P. P. J., Jenkinson P.. Disease complexes involving plant parasitic nematodes and soilborne pathogens. Plant Pathology. 2002;51:683–697. doi: 10.1046/j.1365-3059.2002.00785.x. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
