A Systematic Review and Meta-Analysis of the Efficacy and Safety of Rasagiline or Pramipexole in the Treatment of Early Parkinson's Disease
- PMID: 38264500
- PMCID: PMC10805557
- DOI: 10.1155/2024/8448584
A Systematic Review and Meta-Analysis of the Efficacy and Safety of Rasagiline or Pramipexole in the Treatment of Early Parkinson's Disease
Abstract
Background: Rasagiline or pramipexole monotherapy has been suggested for the management of early Parkinson's disease (PD). The aim of this research was to systematically review the clinical efficacy and safety of rasagiline or pramipexole in early PD (defined as disease duration ≤5 years and Hoehn and Yahr stage of ≤3).
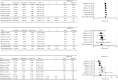
Methods: Randomized controlled trials (RCTs) of rasagiline or pramipexole for early PD published up to September 2021 were retrieved. Outcomes of interest included changes in the Unified Parkinson's Disease Rating Scale (UPDRS) Parts II and III and the incidence of adverse events. Standardized mean difference (SMD), odds ratio (OR), and 95% confidence interval (CI) were calculated, and heterogeneity was measured with the I2 test.
Results: Nine rasagiline and eleven pramipexole RCTs were included. One post hoc analysis of one rasagiline study was included. Five studies for each drug were included in meta-analyses of the UPDRS scores. The rasagiline meta-analysis focused on patients receiving 1 mg/day. Rasagiline and pramipexole significantly improved UPDRS Part II and III scores when compared to placebo. Significant heterogeneity among the studies was present (I2 > 70%). Neither rasagiline nor pramipexole increased the relative risk for any adverse events, serious adverse events, or adverse events leading to withdrawal when compared with placebo.
Conclusion: Applying a Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach to summarize the evidence, we found moderate confidence in the body of evidence for the efficacy of rasagiline or pramipexole in early PD, suggesting further well-designed, multicenter comparative RCTs remain needed.
Copyright © 2024 Pauli Seppänen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources

