Using real-world data to inform dosing strategies of rituximab for pediatric patients with frequent-relapsing or steroid-dependent nephrotic syndrome: a prospective pharmacokinetic-pharmacodynamic study
- PMID: 38264525
- PMCID: PMC10803641
- DOI: 10.3389/fphar.2023.1319744
Using real-world data to inform dosing strategies of rituximab for pediatric patients with frequent-relapsing or steroid-dependent nephrotic syndrome: a prospective pharmacokinetic-pharmacodynamic study
Abstract
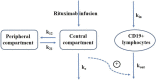
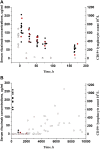
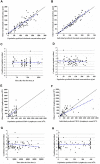
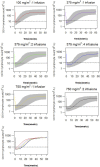
Objectives: Rituximab is frequently used off-label for the treatment of frequent-relapsing/steroid-dependent nephrotic syndrome (FRNS/SDNS). However, the optimal dosing schedules remain undetermined. The objective of this study was to establish a population pharmacokinetic-pharmacodynamic (PK-PD) model in pediatric patients with FRNS/SDNS, and to investigate dosing regimens that provide adequate suppression of B lymphocytes. Methods: A prospective, open-label, single-center study was conducted in Nephrology Department at Children's Hospital of Fudan University, and a two-compartment PK model of rituximab in pediatric FRNS/SDNS has been developed previously by our group. CD19+ lymphocyte count profiles were obtained from these patients. The presence of anti-rituximab antibodies was assessed prior to medication in children who had previously received rituximab or during follow-up at the last sampling point for PK analysis. PK-PD analyses were performed to describe the changes of CD19+ lymphocytes, with rituximab assumed to increase their death rate. Monte Carlo simulation was conducted to evaluate different dosing regimens. Results: In total, 102 measurements of CD19+ lymphocyte counts were available for PK-PD analysis. No detectable levels of anti-rituximab antibodies were observed during the PK follow-up period. A turnover model with saturable stimulatory action of rituximab on the removal of lymphocytes best characterized the relationship between rituximab concentration and CD19+ lymphocyte counts, where the Emax and EC50 were estimated to be 99.6*106/L and 5.87 μg/mL, respectively. Simulations indicated that a single infusion of 750 mg/m2 and 2 infusions of 375 mg/m2 both yielded a 10-week suppression of CD19+ lymphocytes. Conclusion: This study represents a first attempt to quantitatively describe the PK-PD relationship of rituximab in pediatric patients with FRNS/SDNS, and provide a potential pathway for future precision dosing strategy for rituximab therapy. Further clinical studies are warranted to evaluate the efficacy and safety of different dosing schemes.
Keywords: nephrology; pediatric; pharmacodynamics; pharmacokinetics; precision medicine.
Copyright © 2024 Chen, Shen, Xiong, Dong, Xu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor BB declared a past co-authorship with the author HX.
Figures

Similar articles
-
Population Pharmacokinetics of Rituximab in Pediatric Patients With Frequent-Relapsing or Steroid-Dependent Nephrotic Syndrome.Front Pharmacol. 2021 Sep 2;12:725665. doi: 10.3389/fphar.2021.725665. eCollection 2021. Front Pharmacol. 2021. PMID: 34539407 Free PMC article.
-
Study protocol: mycophenolate mofetil as maintenance therapy after rituximab treatment for childhood-onset, complicated, frequently-relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicenter double-blind, randomized, placebo-controlled trial (JSKDC07).BMC Nephrol. 2018 Nov 1;19(1):302. doi: 10.1186/s12882-018-1099-7. BMC Nephrol. 2018. PMID: 30382824 Free PMC article. Clinical Trial.
-
Study protocol: multicenter double-blind, randomized, placebo-controlled trial of rituximab for the treatment of childhood-onset early-stage uncomplicated frequently relapsing or steroid-dependent nephrotic syndrome (JSKDC10 trial).BMC Nephrol. 2019 Aug 2;20(1):293. doi: 10.1186/s12882-019-1470-3. BMC Nephrol. 2019. PMID: 31375087 Free PMC article.
-
Rituximab for childhood refractory nephrotic syndrome.Pediatr Int. 2011 Oct;53(5):617-621. doi: 10.1111/j.1442-200X.2011.03432.x. Pediatr Int. 2011. PMID: 21771179 Review.
-
Rituximab in steroid-sensitive nephrotic syndrome: lessons from clinical trials.Pediatr Nephrol. 2018 Sep;33(9):1449-1455. doi: 10.1007/s00467-017-3746-9. Epub 2017 Jul 17. Pediatr Nephrol. 2018. PMID: 28717938 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

