A reappraisal of the default mode and frontoparietal networks in the common marmoset brain
- PMID: 38264540
- PMCID: PMC10803424
- DOI: 10.3389/fnimg.2023.1345643
A reappraisal of the default mode and frontoparietal networks in the common marmoset brain
Abstract
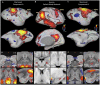
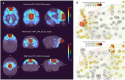
In recent years the common marmoset homolog of the human default mode network (DMN) has been a hot topic of discussion in the marmoset research field. Previously, the posterior cingulate cortex regions (PGM, A19M) and posterior parietal cortex regions (LIP, MIP) were defined as the DMN, but some studies claim that these form the frontoparietal network (FPN). We restarted from a neuroanatomical point of view and identified two DMN candidates: Comp-A (which has been called both the DMN and FPN) and Comp-B. We performed GLM analysis on auditory task-fMRI and found Comp-B to be more appropriate as the DMN, and Comp-A as the FPN. Additionally, through fingerprint analysis, a DMN and FPN in the tasking human was closer to the resting common marmoset. The human DMN appears to have an advanced function that may be underdeveloped in the common marmoset brain.
Keywords: common marmoset; default mode network; fMRI; frontoparietal network; general linear model (GLM); independent component analysis (ICA).
Copyright © 2024 Okuno, Ichinohe and Woodward.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Default mode network scaffolds immature frontoparietal network in cognitive development.Cereb Cortex. 2023 Apr 25;33(9):5251-5263. doi: 10.1093/cercor/bhac414. Cereb Cortex. 2023. PMID: 36320154 Free PMC article.
-
Investigating intrinsic connectivity networks using simultaneous BOLD and CBF measurements.Neuroimage. 2014 Oct 1;99:111-21. doi: 10.1016/j.neuroimage.2014.05.042. Epub 2014 May 23. Neuroimage. 2014. PMID: 24857826
-
Large-scale brain network connectivity underlying creativity in resting-state and task fMRI: Cooperation between default network and frontal-parietal network.Biol Psychol. 2018 May;135:102-111. doi: 10.1016/j.biopsycho.2018.03.005. Epub 2018 Mar 13. Biol Psychol. 2018. PMID: 29548807
-
Resting-state fMRI functional connectivity and mindfulness in clinical and non-clinical contexts: A review and synthesis.Neurosci Biobehav Rev. 2022 Apr;135:104583. doi: 10.1016/j.neubiorev.2022.104583. Epub 2022 Feb 22. Neurosci Biobehav Rev. 2022. PMID: 35202647 Free PMC article. Review.
-
Subcortical control of the default mode network: Role of the basal forebrain and implications for neuropsychiatric disorders.Brain Res Bull. 2022 Jul;185:129-139. doi: 10.1016/j.brainresbull.2022.05.005. Epub 2022 May 11. Brain Res Bull. 2022. PMID: 35562013 Free PMC article. Review.
Cited by
-
A Novel Directed Seed-Based Connectivity Analysis Toolbox Applied to Human and Marmoset Resting-State FMRI.J Neurosci. 2024 Nov 6;44(45):e0389242024. doi: 10.1523/JNEUROSCI.0389-24.2024. J Neurosci. 2024. PMID: 39299799 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

