OTUB1-mediated inhibition of ubiquitination: a growing list of effectors, multiplex mechanisms, and versatile functions
- PMID: 38264570
- PMCID: PMC10803509
- DOI: 10.3389/fmolb.2023.1261273
OTUB1-mediated inhibition of ubiquitination: a growing list of effectors, multiplex mechanisms, and versatile functions
Abstract
Protein ubiquitination plays a pivotal role in protein homeostasis. Ubiquitination may regulate the stability, activity, protein-protein interaction, and localization of a protein. Ubiquitination is subject to regulation by two groups of counteracting enzymes, the E3 ubiquitin ligases and deubiquitinases. Consistently, deubiquitinases are involved in essentially all biological processes. OTUB1, an OTU-family deubiquitinase, is a critical regulator of development, cancer, DNA damage response, and immune response. OTUB1 antagonizes the ubiquitination of a wide-spectrum of proteins through at least two different mechanisms. Besides direct deubiquitination, OTUB1 can also inhibit ubiquitination by non-canonically blocking ubiquitin transfer from certain ubiquitin-conjugases (E2). In this review, we start with a general background of protein ubiquitination and deubiquitination. Next, we introduce the basic characteristics of OTUB1 and then elaborate on the updated biological functions of OTUB1. Afterwards, we discuss potential mechanisms underlying the versatility and specificity of OTUB1 functions. In the end, we discuss the perspective that OTUB1 can be a potential therapeutic target for cancer.
Keywords: OTUB1; cancer; degradation; deubiquitinase; ubiquitin-conjugating enzyme; ubiquitination.
Copyright © 2024 Wu, Sun and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
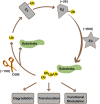


 denotes ubiquitin.
denotes ubiquitin.

 denotes a promoting effect while
denotes a promoting effect while  denotes an inhibiting effect. (B) By inhibiting ubiquitination of P53 and MDMX, OTUB1 can promote cellular apoptosis upon DNA damage.
denotes an inhibiting effect. (B) By inhibiting ubiquitination of P53 and MDMX, OTUB1 can promote cellular apoptosis upon DNA damage.  denotes a promoting effect while
denotes a promoting effect while  denotes an inhibiting effect.
denotes an inhibiting effect.

Similar articles
-
Deubiquitinating enzyme regulation of the p53 pathway: A lesson from Otub1.World J Biol Chem. 2014 May 26;5(2):75-84. doi: 10.4331/wjbc.v5.i2.75. World J Biol Chem. 2014. PMID: 24920999 Free PMC article. Review.
-
OTUB1 non-catalytically stabilizes the E2 ubiquitin-conjugating enzyme UBE2E1 by preventing its autoubiquitination.J Biol Chem. 2018 Nov 23;293(47):18285-18295. doi: 10.1074/jbc.RA118.004677. Epub 2018 Oct 3. J Biol Chem. 2018. PMID: 30282802 Free PMC article.
-
OTUB1 stabilizes mismatch repair protein MSH2 by blocking ubiquitination.J Biol Chem. 2021 Jan-Jun;296:100466. doi: 10.1016/j.jbc.2021.100466. Epub 2021 Feb 26. J Biol Chem. 2021. PMID: 33640455 Free PMC article.
-
The ubiquitin-like modifier FAT10 stimulates the activity of deubiquitylating enzyme OTUB1.J Biol Chem. 2019 Mar 22;294(12):4315-4330. doi: 10.1074/jbc.RA118.005406. Epub 2019 Feb 4. J Biol Chem. 2019. PMID: 30718280 Free PMC article.
-
Otubain 1: a non-canonical deubiquitinase with an emerging role in cancer.Endocr Relat Cancer. 2019 Jan 1;26(1):R1-R14. doi: 10.1530/ERC-18-0264. Endocr Relat Cancer. 2019. PMID: 30400005 Free PMC article. Review.
Cited by
-
Mechanical Load-Induced Upregulation of Talin2 through Non-Canonical Deubiquitination of OTUB1 Drives Facet Joint Osteoarthritis Pathogenesis.Adv Sci (Weinh). 2025 Jul;12(25):e2501046. doi: 10.1002/advs.202501046. Epub 2025 Apr 25. Adv Sci (Weinh). 2025. PMID: 40279639 Free PMC article.
-
OTUB1 antagonizes TRIM21 to induce deubiquitination of SPHK1 and promote the progression of hepatocellular carcinoma.Oncogene. 2025 Aug 30. doi: 10.1038/s41388-025-03556-0. Online ahead of print. Oncogene. 2025. PMID: 40885853
-
Mechanism of Curcumol Targeting the OTUB1/TGFBI Ubiquitination Pathway in the Inhibition of Angiogenesis in Colon Cancer.Int J Mol Sci. 2025 May 21;26(10):4899. doi: 10.3390/ijms26104899. Int J Mol Sci. 2025. PMID: 40430059 Free PMC article.
-
Deubiquitinases as novel therapeutic targets for diseases.MedComm (2020). 2024 Dec 13;5(12):e70036. doi: 10.1002/mco2.70036. eCollection 2024 Dec. MedComm (2020). 2024. PMID: 39678489 Free PMC article. Review.
-
Bacterial ubiquitin ligases hijack the host deubiquitinase OTUB1 to inhibit MTORC1 signaling and promote autophagy.Autophagy. 2024 Sep;20(9):1968-1983. doi: 10.1080/15548627.2024.2353492. Epub 2024 May 31. Autophagy. 2024. PMID: 38818749 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

