The role of miRNAs in T helper cell development, activation, fate decisions and tumor immunity
- PMID: 38264670
- PMCID: PMC10803515
- DOI: 10.3389/fimmu.2023.1320305
The role of miRNAs in T helper cell development, activation, fate decisions and tumor immunity
Abstract
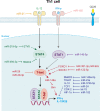
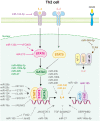
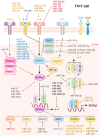
T helper (Th) cells are central members of adaptive immunity and comprise the last line of defense against pathogen infection and malignant cell invasion by secreting specific cytokines. These cytokines then attract or induce the activation and differentiation of other immune cells, including antibody-producing B cells and cytotoxic CD8+ T cells. Therefore, the bidirectional communication between Th cells and tumor cells and their positioning within the tumor microenvironment (TME), especially the tumor immune microenvironment (TIME), sculpt the tumor immune landscape, which affects disease initiation and progression. The type, number, and condition of Th cells in the TME and TIME strongly affect tumor immunity, which is precisely regulated by key effectors, such as granzymes, perforins, cytokines, and chemokines. Moreover, microRNAs (miRNAs) have emerged as important regulators of Th cells. In this review, we discuss the role of miRNAs in regulating Th cell mediated adaptive immunity, focusing on the development, activation, fate decisions, and tumor immunity.
Keywords: activation; development; fate decisions; miRNAs; T helper cell; tumor immunity; tumor microenvironment.
Copyright © 2024 Xu, Chen, Chang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Janus or Hydra: The Many Faces of T Helper Cells in the Human Tumour Microenvironment.Adv Exp Med Biol. 2020;1224:35-51. doi: 10.1007/978-3-030-35723-8_3. Adv Exp Med Biol. 2020. PMID: 32036603 Review.
-
Dysregulation of helper T lymphocytes in esophageal squamous cell carcinoma (ESCC) patients is highly associated with aberrant production of miR-21.Immunol Res. 2019 Jun;67(2-3):212-222. doi: 10.1007/s12026-019-09079-7. Immunol Res. 2019. PMID: 31278653
-
IL-18-primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment.Cancer Res. 2013 Aug 1;73(15):4653-62. doi: 10.1158/0008-5472.CAN-12-4366. Epub 2013 Jun 12. Cancer Res. 2013. PMID: 23761327 Free PMC article.
-
Posttranscriptional regulation of T helper cell fate decisions.J Cell Biol. 2018 Aug 6;217(8):2615-2631. doi: 10.1083/jcb.201708075. Epub 2018 Apr 23. J Cell Biol. 2018. PMID: 29685903 Free PMC article. Review.
-
Helper T cell differentiation.Cell Mol Immunol. 2019 Jul;16(7):634-643. doi: 10.1038/s41423-019-0220-6. Epub 2019 Mar 12. Cell Mol Immunol. 2019. PMID: 30867582 Free PMC article. Review.
Cited by
-
miRNA-Mediated Regulation of Gene Expression During Early Activation in Jurkat Cells.bioRxiv [Preprint]. 2025 Jun 17:2025.06.15.659805. doi: 10.1101/2025.06.15.659805. bioRxiv. 2025. PMID: 40667200 Free PMC article. Preprint.
-
Exosome-derived microRNAs: emerging players in vitiligo.Front Immunol. 2024 Jul 8;15:1419660. doi: 10.3389/fimmu.2024.1419660. eCollection 2024. Front Immunol. 2024. PMID: 39040109 Free PMC article. Review.
-
Targeting inflammation in cancer therapy: from mechanistic insights to emerging therapeutic approaches.J Transl Med. 2025 May 26;23(1):588. doi: 10.1186/s12967-025-06583-3. J Transl Med. 2025. PMID: 40420174 Free PMC article. Review.
-
G-Quadruplex Conformational Switching for miR-155-3p Detection Using a Ligand-Based Fluorescence Approach.Biomolecules. 2025 Mar 13;15(3):410. doi: 10.3390/biom15030410. Biomolecules. 2025. PMID: 40149946 Free PMC article.
-
The Role of Non-coding RNAs in Tumorigenesis, Diagnosis/Prognosis, and Therapeutic Strategies for Cutaneous Melanoma.Methods Mol Biol. 2025;2883:79-107. doi: 10.1007/978-1-0716-4290-0_4. Methods Mol Biol. 2025. PMID: 39702705 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

