Functional activation of pyruvate dehydrogenase in human brain using hyperpolarized [1-13 C]pyruvate
- PMID: 38265104
- PMCID: PMC10950523
- DOI: 10.1002/mrm.30015
Functional activation of pyruvate dehydrogenase in human brain using hyperpolarized [1-13 C]pyruvate
Abstract
Purpose: Pyruvate, produced from either glucose, glycogen, or lactate, is the dominant precursor of cerebral oxidative metabolism. Pyruvate dehydrogenase (PDH) flux is a direct measure of cerebral mitochondrial function and metabolism. Detection of [13 C]bicarbonate in the brain from hyperpolarized [1-13 C]pyruvate using carbon-13 (13 C) MRI provides a unique opportunity for assessing PDH flux in vivo. This study is to assess changes in cerebral PDH flux in response to visual stimuli using in vivo 13 C MRS with hyperpolarized [1-13 C]pyruvate.
Methods: From seven sedentary adults in good general health, time-resolved [13 C]bicarbonate production was measured in the brain using 90° flip angles with minimal perturbation of its precursors, [1-13 C]pyruvate and [1-13 C]lactate, to test the hypothesis that the appearance of [13 C]bicarbonate signals in the brain reflects the metabolic changes associated with neuronal activation. With a separate group of healthy participants (n = 3), the likelihood of the bolus-injected [1-13 C]pyruvate being converted to [1-13 C]lactate prior to decarboxylation was investigated by measuring [13 C]bicarbonate production with and without [1-13 C]lactate saturation.
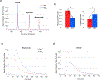
Results: In the course of visual stimulation, the measured [13 C]bicarbonate signal normalized to the total 13 C signal in the visual cortex increased by 17.1% ± 15.9% (p = 0.017), whereas no significant change was detected in [1-13 C]lactate. Proton BOLD fMRI confirmed the regional activation in the visual cortex with the stimuli. Lactate saturation decreased bicarbonate-to-pyruvate ratio by 44.4% ± 9.3% (p < 0.01).
Conclusion: We demonstrated the utility of 13 C MRS with hyperpolarized [1-13 C]pyruvate for assessing the activation of cerebral PDH flux via the detection of [13 C]bicarbonate production.
Keywords: bicarbonate; brain activation; dissolution dynamic nuclear polarization; fMRS; hyperpolarized pyruvate; pyruvate dehydrogenase.
© 2024 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
Figures



References
-
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. - PubMed
-
- Brown GG, Perthen JE, Liu TT, Buxton RB. A primer on functional magnetic resonance imaging. Neuropsychol Rev. 2007;17(2):107–125. - PubMed
-
- Reed LJ. Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Curr Top Cell Regul. 1981;18(7):95–106. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

