Lewy body dementia: Overcoming barriers and identifying solutions
- PMID: 38265159
- PMCID: PMC10942666
- DOI: 10.1002/alz.13674
Lewy body dementia: Overcoming barriers and identifying solutions
Abstract
Despite its high prevalence among dementias, Lewy body dementia (LBD) remains poorly understood with a limited, albeit growing, evidence base. The public-health burden that LBD imposes is worsened by overlapping pathologies, which contribute to misdiagnosis, and lack of treatments. For this report, we gathered and analyzed public-domain information on advocacy, funding, research outputs, and the therapeutic pipeline to identify gaps in each of these key elements. To further understand the current gaps, we also conducted interviews with leading experts in regulatory/governmental agencies, LBD advocacy, academic research, and biopharmaceutical research, as well as with funding sources. We identified wide gaps across the entire landscape, the most critical being in research. Many of the experts participated in a workshop to discuss the prioritization of research areas with a view to accelerating therapeutic development and improving patient care. This white paper outlines the opportunities for bridging the major LBD gaps and creates the framework for collaboration in that endeavor. HIGHLIGHTS: A group representing academia, government, industry, and consulting expertise was convened to discuss current progress in Dementia with Lewy Body care and research. Consideration of expert opinion,natural language processing of the literature as well as publicly available data bases, and Delphi inspired discussion led to a proposed consensus document of priorities for the field.
Keywords: Lewy body dementia; funding sources; natural language processing; research trends.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. At the time of the research, K.A., W.B., H.K., M.K., S.O.M., V.P., M.S.R., J.C.S., and L.W. were employees of Boston Consulting Group which funded the research for this article through BCG's health care practice. B.F.B. is an investigator for clinical trials sponsored by Biogen, Alector, EIP Pharma, Cognition Therapeutics, and Transposon; a Scientific Advisory Board member for the Tau Consortium, AFTD, LBDA, and GE HealthCare; and serves as a member of the Data Safety Monitoring Board for a clinical trial on mesenchymal stem cells in MSA. J.H.C. is a former employee of Novartis, current employee and stockholder of Latus Bio, Inc., and serves as a member of the Comprehensive Center for X‐Linked Parkinsons Dementia (CCXD) Scientific Advisory Board. M.D. and M.C.I. are employees of Eisai, Inc., which has drug development programs in neurodegenerative disease. D.G. is a consultant for GE Healthcare. J.E.G. provides consultations to Biogen, BMS, Eisai, Eli Lilly, GE Healthcare, and Lundbeck, serves on the advisory board for Passage Bio, is the Chief Scientific Officer and stockholder at Cognivue which develops cognitive assessment tools. S.G. consults for EIP Pharma and serves on the advisory boards of Hillhurst Biopharmaceuticals, EIP Pharma, and the LBDA RCOE. K.K. consults for Biogen and serves on a Data Safety Monitoring Board at Takeda. A.K., holds stock in Sage Therapeutics and Delix Therapeutics. J.B.L. serves as an advisor for LBDA and the Cleveland Alzheimer's Association Chapter Board. J.T.O. consults for Biogen and Roche, serves on advisory boards for TauRx, NovoNordisk, Lilly, and the Lewy Body Society, as well as chairs the Research Strategy Council for the Alzheimer's Society. S.W.S. serves on the Scientific Advisory Council of the Lewy Body Dementia Association; receives research support from Cerevel Therapeutics; and serves on the scientific advisory council for the LBDA and Multiple System Atrophy Coalition. R.S. consults for AbbVie, AC Immune, Acumen, Alector, Alnylam, Bristol Myers Squibb, Genentech, Janssen, JOMDD, Nervgen, Neuraly, Neurocentria, Oligomerix, Prothena, Renew, Roche, Shionogi, Vigil Neuroscience, Ionis, and Vaxxinity. A.T. is an employee of the Lewy Body Dementia Association. J.P.T. consults for EIP Pharma, Kyowa‐Kirin, and Sosei‐Heptares; and serves on the scientific advisory committee for the LBDA and the Lewy Body Society. R.A.W. provided freelance medical writing services for this manuscript. The following authors have no additional disclosures (funding support related to this manuscript is listed above): E.B., L.B., T.J.F., B.H., I.M., P.J.M., T.M., and S.R. Author disclosures are available in the S2.
Figures



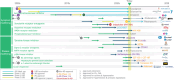
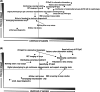
References
-
- Lippa CF, Duda JE, Grossman M, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812‐819. - PubMed
-
- Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44:673‐683. - PubMed
-
- Jellinger KA. Depression in dementia with Lewy bodies: a critical update. J Neural Transm. 2023;130(10):1207‐1218 - PubMed
Publication types
MeSH terms
Grants and funding
- K99 AG073453/AG/NIA NIH HHS/United States
- U01NS100620/GF/NIH HHS/United States
- U01 NS100610/NS/NINDS NIH HHS/United States
- P30AG062677/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- U01NS100620/AG/NIA NIH HHS/United States
- ZIANS003154/GF/NIH HHS/United States
- U19AG071754/GF/NIH HHS/United States
- Z01 AG000733/ImNIH/Intramural NIH HHS/United States
- ZIANS003154/NS/NINDS NIH HHS/United States
- ZIA NS003154/ImNIH/Intramural NIH HHS/United States
- P30 AG072959/AG/NIA NIH HHS/United States
- K99AG073453/AG/NIA NIH HHS/United States
- U01 AG073323/AG/NIA NIH HHS/United States
- U01NS100610/GF/NIH HHS/United States
- U19 AG071754/AG/NIA NIH HHS/United States
- U19AG071754/AG/NIA NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- P30AG062677/GF/NIH HHS/United States
- P30AG072959/GF/NIH HHS/United States
- U01 NS100620/NS/NINDS NIH HHS/United States
- U01AG0733/GF/NIH HHS/United States

