Quantitative MRI reveals heterogeneous impacts of treatment on diseased bone marrow in a mouse model of myelofibrosis
- PMID: 38265182
- PMCID: PMC10997455
- DOI: 10.1002/mrm.30016
Quantitative MRI reveals heterogeneous impacts of treatment on diseased bone marrow in a mouse model of myelofibrosis
Abstract
Purpose: Analyzing bone marrow in the hematologic cancer myelofibrosis requires endpoint histology in mouse models and bone marrow biopsies in patients. These methods hinder the ability to monitor therapy over time. Preclinical studies typically begin treatment before mice develop myelofibrosis, unlike patients who begin therapy only after onset of disease. Using clinically relevant, quantitative MRI metrics allowed us to evaluate treatment in mice with established myelofibrosis.
Methods: We used chemical shift-encoded fat imaging, DWI, and magnetization transfer sequences to quantify bone marrow fat, cellularity, and macromolecular components in a mouse model of myelofibrosis. We monitored spleen volume, the established imaging marker for treatment, with anatomic MRI. After confirming bone marrow disease by MRI, we randomized mice to treatment with an approved drug (ruxolitinib or fedratinib) or an investigational agent, navitoclax, for 33 days. We measured the effects of therapy over time with bone marrow and spleen MRI.
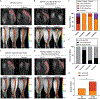
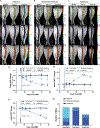
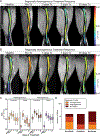
Results: All treatments produced heterogeneous responses with improvements in bone marrow evident in subsets of individual mice in all treatment groups. Reductions in spleen volume commonly occurred without corresponding improvement in bone marrow. MRI revealed patterns associated with effective and ineffective responses to treatment in bone marrow and identified regional variations in efficacy within a bone.
Conclusions: Quantitative MRI revealed modest, heterogeneous improvements in bone marrow disease when treating mice with established myelofibrosis. These results emphasize the value of bone marrow MRI to assess treatment in preclinical models and the potential to advance clinical trials for patients.
Keywords: MRI; bone marrow; myelofibrosis; treatment response.
© 2024 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
Conflicts of Interest
T.L.C. and B.D.R. have patents and may receive royalties from the University of Michigan related to the use of DWI for cancer treatment assessment. B.D.R. has a financial interest in Imbio, a company that has licensed this technology.
Figures


References
MeSH terms
Grants and funding
- R50 CA221807/CA/NCI NIH HHS/United States
- R01 CA190299/CA/NCI NIH HHS/United States
- R21 AI173559/AI/NIAID NIH HHS/United States
- R61 CA281657/CA/NCI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- R01 CA238042/CA/NCI NIH HHS/United States
- R01 CA238023/CA/NCI NIH HHS/United States
- R35 CA197701/CA/NCI NIH HHS/United States
- R01 CA166104/GF/NIH HHS/United States
- U24 CA237683/CA/NCI NIH HHS/United States
- R01 CA190299/GF/NIH HHS/United States
- U01 CA210152/CA/NCI NIH HHS/United States
- R50CA221807/GF/NIH HHS/United States
- P30CA046592/GF/NIH HHS/United States
- R01CA238042/GF/NIH HHS/United States
- U24 CA237683/GF/NIH HHS/United States
- U01CA210152/GF/NIH HHS/United States
- R35CA197701/GF/NIH HHS/United States
- U01 CA166104/CA/NCI NIH HHS/United States
- R01 CA238023/GF/NIH HHS/United States
- R37CA222563/GF/NIH HHS/United States
- R33 CA225549/CA/NCI NIH HHS/United States
- R37 CA222563/CA/NCI NIH HHS/United States
- R33CA225549/GF/NIH HHS/United States

