Comparison of plasma biomarkers and amyloid PET for predicting memory decline in cognitively unimpaired individuals
- PMID: 38265198
- PMCID: PMC10984437
- DOI: 10.1002/alz.13651
Comparison of plasma biomarkers and amyloid PET for predicting memory decline in cognitively unimpaired individuals
Abstract
Background: We compared the ability of several plasma biomarkers versus amyloid positron emission tomography (PET) to predict rates of memory decline among cognitively unimpaired individuals.
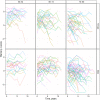
Methods: We studied 645 Mayo Clinic Study of Aging participants. Predictor variables were age, sex, education, apolipoprotein E (APOE) ε4 genotype, amyloid PET, and plasma amyloid beta (Aβ)42/40, phosphorylated tau (p-tau)181, neurofilament light (NfL), glial fibrillary acidic protein (GFAP), and p-tau217. The outcome was a change in a memory composite measure.
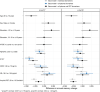
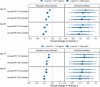
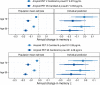
Results: All plasma biomarkers, except NfL, were associated with mean memory decline in models with individual biomarkers. However, amyloid PET and plasma p-tau217, along with age, were key variables independently associated with mean memory decline in models combining all predictors. Confidence intervals were narrow for estimates of population mean prediction, but person-level prediction intervals were wide.
Discussion: Plasma p-tau217 and amyloid PET provide useful information about predicting rates of future cognitive decline in cognitively unimpaired individuals at the population mean level, but not at the individual person level.
Keywords: amyloid PET; cognitive decline; cognitive decline and Alzheimer's disease; plasma biomarkers and Alzheimer's disease.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
CR Jack receives funding from the NIH and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic. A Algeciras‐Schimnich has participated in advisory boards for Roche Diagnostics, Fujirebio Diagnostics, and Siemens Healthineers. DJ Figdore, VK Ramanan, PM Cogswell, SD Weigand, HJ Wiste, and J Fields report no disclosures. TM Therneau receives NIH support. MM Mielke receives research support from the NIH and DOD and has consulted for Biogen, Brain Protection Company, LabCorp, Lilly, Merck, Roche, Siemens Healthineers, and Sunbird Bio. MM Machulda and ML Senjem report no disclosures. DS Knopman serves on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer Network Treatment Unit study. He served on a Data Safety monitoring Board for a tau therapeutic for Biogen (until 2021) but received no personal compensation. He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He has served as a consultant for Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences but receives no personal compensation. He attended an Eisai advisory board meeting for lecanemab on December 2, 2022, but received no compensation. He receives funding from the NIH. J Graff‐Radford receives funding from the NIH. He is an investigator in clinical trials sponsored by Biogen, Eisai, and the University of Southern California. VJ Lowe consults for Bayer Schering Pharma, Piramal Life Sciences, Eisai, Inc., AVID Radiopharmaceuticals, and Merck Research, and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH (NIA, NCI). P Vemuri receives funding from the NIH. CG Schwarz receives funding from the NIH. RC Petersen has consulted for Roche, Inc., Genentech, Inc., Eli Lilly, Inc., Nestle, Inc., and Eisai, Inc.; has served on a DSMB for Genentech, Inc., and receives royalties from Oxford University Press for
Figures

References
-
- Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho‐tau181 in the Alzheimer's Disease Neuroimaging Initiative. Mol Psychiatry. 2020. Online ahead of print. - PubMed
-
- Palmqvist S, Tideman P, Cullen N, et al. Prediction of future Alzheimer's disease dementia using plasma phospho‐tau combined with other accessible measures. Nat Med. 2021;27(6):1034‐1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

