High molecular/low acetylated chitosans reduce adhesion of Campylobacter jejuni to host cells by blocking JlpA
- PMID: 38265503
- PMCID: PMC10810038
- DOI: 10.1007/s00253-024-13000-0
High molecular/low acetylated chitosans reduce adhesion of Campylobacter jejuni to host cells by blocking JlpA
Abstract
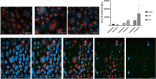
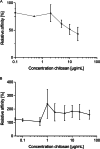
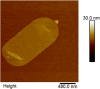
Infections caused by Campylobacter spp. are a major cause of severe enteritis worldwide. Multifactorial prevention strategies are necessary to reduce the prevalence of Campylobacter. In particular, antiadhesive strategies with specific inhibitors of early host-pathogen interaction are promising approaches to reduce the bacterial load. An in vitro flow cytometric adhesion assay was established to study the influence of carbohydrates on the adhesion of C. jejuni to Caco-2 cells. Chitosans with a high degree of polymerization and low degree of acetylation were identified as potent antiadhesive compounds, exerting significant reduction of C. jejuni adhesion to Caco-2 cells at non-toxic concentrations. Antiadhesive and also anti-invasive effects were verified by confocal laser scanning microscopy. For target identification, C. jejuni adhesins FlpA and JlpA were expressed in Escherichia coli ArcticExpress, and the influence of chitosan on binding to fibronectin and HSP90α, respectively, was investigated. While no effects on FlpA binding were found, a strong inhibition of JlpA-HSP90α binding was observed. To simulate real-life conditions, chicken meat was inoculated with C. jejuni, treated with antiadhesive chitosan, and the bacterial load was quantified. A strong reduction of C. jejuni load was observed. Atomic force microscopy revealed morphological changes of C. jejuni after 2 h of chitosan treatment, indicating disturbance of the cell wall and sacculi formation by electrostatic interaction of positively charged chitosan with the negatively charged cell surface. In conclusion, our data indicate promising antiadhesive and anti-invasive potential of high molecular weight, strongly de-acetylated chitosans for reducing C. jejuni load in livestock and food production. KEY POINTS: • Antiadhesive effects of chitosan with high DP/low DA against C. jejuni to host cells • Specific targeting of JlpA/Hsp90α interaction by chitosan • Meat treatment with chitosan reduces C. jejuni load.
Keywords: Adhesion; Atomic force microscopy; Campylobacter jejuni; Chitosan; JlpA; Sacculus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Baek YJ, Song JE, Kim EJ, Choi H, Sohn Y, Jeon YD, Lee EH, Ahn JY, Jeong SJ, Ku NS, Choi JY, Yeom J-S, Song YG, Kim JH (2023) Trends, clinical characteristics, antimicrobial susceptibility patterns, and outcomes of Campylobacter bacteraemia: A multicentre retrospective study. Infection. 10.1007/s15010-023-02118-4 - PubMed
-
- Bakhshi B, Barzelighi HM, Daraei B (2020) The anti-adhesive and anti-invasive effects of recombinant azurin on the interaction between enteric pathogens (invasive/non-invasive) and Caco-2 cells. Microb Pathogenesis 147:104246. 10.1016/j.micpath.2020.104246 - PubMed
-
- Bensch K, Tiralongo J, Schmidt K, Matthias A, Bone KM, Lehmann R, Tiralongo E (2011) Investigations into the antiadhesive activity of herbal extracts against Campylobacter jejuni. Phytother Res 25:1125–1132. 10.1002/ptr.3384 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

