Characteristics of splenic PD-1+ γδT cells in Plasmodium yoelii nigeriensis infection
- PMID: 38265549
- PMCID: PMC11217126
- DOI: 10.1007/s12026-023-09441-w
Characteristics of splenic PD-1+ γδT cells in Plasmodium yoelii nigeriensis infection
Erratum in
-
Correction to: Characteristics of splenic PD-1+ γδT cells in Plasmodium yoelii nigeriensis infection.Immunol Res. 2024 Jun;72(3):395. doi: 10.1007/s12026-024-09469-6. Immunol Res. 2024. PMID: 38421495 Free PMC article. No abstract available.
Abstract
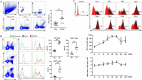
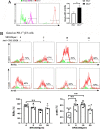
Although the functions of programmed death-1 (PD-1) on αβ T cells have been extensively reported, a role for PD-1 in regulating γδT cell function is only beginning to emerge. Here, we investigated the phenotypic and functional characteristics of PD-1-expressing γδT cells, and the molecular mechanism was also explored in the Plasmodium yoelii nigeriensis (P. yoelii NSM)-infected mice. Flow cytometry and single-cell RNA sequencing (scRNA-seq) were performed. An inverse agonist of RORα, SR3335, was used to investigate the role of RORα in regulating PD-1+ γδT cells. The results indicated that γδT cells continuously upregulated PD-1 expression during the infection period. Higher levels of CD94, IL-10, CX3CR1, and CD107a; and lower levels of CD25, CD69, and CD127 were found in PD-1+ γδT cells from infected mice than in PD-1- γδT cells. Furthermore, GO enrichment analysis revealed that the marker genes in PD-1+ γδT cells were involved in autophagy and processes utilizing autophagic mechanisms. ScRNA-seq results showed that RORα was increased significantly in PD-1+ γδT cells. GSEA identified that RORα was mainly involved in the regulation of I-kappaB kinase/NF-κB signaling and the positive regulation of cytokine production. Consistent with this, PD-1-expressing γδT cells upregulated RORα following Plasmodium yoelii infection. Additionally, in vitro studies revealed that higher levels of p-p65 were found in PD-1+ γδT cells after treatment with a RORα selective synthetic inhibitor. Collectively, these data suggest that RORα-mediated attenuation of NF-κB signaling may be fundamental for PD-1-expressing γδT cells to modulate host immune responses in the spleen of Plasmodium yoelii nigeriensis-infected C57BL/6 mice, and it requires further investigation.
Keywords: Plasmodium yoelii nigeriensis NSM; NF-κB; PD-1; RORα; γδT cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Properties and Roles of γδT Cells in Plasmodium yoelii nigeriensis NSM Infected C57BL/6 Mice.Front Cell Infect Microbiol. 2022 Jan 20;11:788546. doi: 10.3389/fcimb.2021.788546. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35127555 Free PMC article.
-
Characterization of γδT cells in lung of Plasmodium yoelii-infected C57BL/6 mice.Malar J. 2021 Feb 15;20(1):89. doi: 10.1186/s12936-021-03619-z. Malar J. 2021. PMID: 33588839 Free PMC article.
-
Characteristics of γδTCR on myeloid cells from C57BL/6 mice with Plasmodium yoelii nigeriensis infection.Mol Biochem Parasitol. 2023 Feb;253:111540. doi: 10.1016/j.molbiopara.2022.111540. Epub 2022 Dec 9. Mol Biochem Parasitol. 2023. PMID: 36509228
-
Immunological characteristics of CD103+CD8+ Tc cells in the liver of C57BL/6 mouse infected with plasmodium NSM.Parasitol Res. 2023 Nov;122(11):2513-2524. doi: 10.1007/s00436-023-07950-z. Epub 2023 Sep 14. Parasitol Res. 2023. PMID: 37707607
-
Gamma/Delta T Cells and Their Role in Protection Against Malaria.Front Immunol. 2018 Dec 20;9:2973. doi: 10.3389/fimmu.2018.02973. eCollection 2018. Front Immunol. 2018. PMID: 30619330 Free PMC article. Review.
Cited by
-
Roles of retinoic acid-related orphan receptor α in high glucose-induced cardiac fibroblasts proliferation.Front Pharmacol. 2025 Jan 30;16:1539690. doi: 10.3389/fphar.2025.1539690. eCollection 2025. Front Pharmacol. 2025. PMID: 39950114 Free PMC article.
References
-
- Daily JP, Minuti A, Khan N. Diagnosis, treatment, and prevention of malaria in the US: a review. JAMA. 2022;328(5):460–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

