Real-world use of multigene signatures in early breast cancer: differences to clinical trials
- PMID: 38265569
- PMCID: PMC11062950
- DOI: 10.1007/s10549-023-07227-0
Real-world use of multigene signatures in early breast cancer: differences to clinical trials
Abstract
Purpose: In Italy, Lombardy was the first region to reimburse multigene assays (MGAs) for patients otherwise candidates for chemotherapy. This is a real-world experience of MGAs usage in six referral cancer centers in Lombardy.
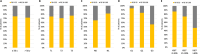
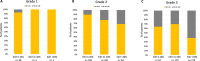
Methods: Among MGAs, Oncotype DX (RS) was used in 97% of cases. Consecutive patients tested with Oncotype DX from July 2020 to July 2022 were selected. The distribution of clinicopathologic features by RS groups (low RS: 0-25, high RS: 26-100) was assessed using chi-square and compared with those of the TAILORx and RxPONDER trials.
Results: Out of 1,098 patients identified, 73% had low RS. Grade and Ki67 were associated with RS (p < 0.001). In patients with both G3 and Ki67 > 30%, 39% had low RS, while in patients with both G1 and Ki67 < 20%, 7% had high RS. The proportion of low RS in node-positive patients was similar to that in RxPONDER (82% vs 83%), while node-negative patients with low RS were significantly less than in TAILORx (66% vs 86%, p < 0.001). The distribution of Grade was different from registration trials, with more G3 and fewer G1 (38% and 3%) than in TAILORx (18% and 27%) and RxPONDER (10% and 24%) (p < 0.001). Patients ≤ 50 years were overrepresented in this series (41%) than in TAILORx and RxPONDER (31% and 24%, respectively) (p < 0.001) and, among them, 42% were node positive.
Conclusions: In this real-world series, Oncotype DX was the test almost exclusively used. Despite reimbursement being linked to pre-test chemotherapy recommendation, almost 3/4 patients resulted in the low-RS group. The significant proportion of node-positive patients ≤ 50 years tested indicates that oncologists considered Oncotype DX informative also in this population.
Keywords: Adjuvant therapy; ER+/HER2− early breast cancer; Multigene assays; Oncotype DX.
© 2024. The Author(s).
Conflict of interest statement
LL has served on the advisory boards for: Lilly, Exact Sciences, AstraZeneca, Italfarmaco and Daiichi Sankyo; has received consulting fee from: Exact Sciences, Helsinn and Eisai; honoraria for speakers’ bureaus from: Gilead and Exact Sciences; support for travel, accommodations, expenses from: Lilly and Gilead. RDS has served on the advisory boards for: Novartis, Lilly, Istituto Clinico Gentili, Ipsen, Amgen, EISAI. AV reports honoraria from Roche and Lilly ELS has received consulting fee and served on the advisory boards for: Exact Sciences, Seagen. EM has received consulting fee and served on the advisory boards for: Exact Sciences, EISAI, MSD Oncology, Daiichi Sankyo/Astra Zeneca, Pfizer, Seagen; support for travel, accommodations, expenses from: Roche, Pfizer, Lilly, Novartis. EGR has received advisory fee from AstraZeneca, Exact Sciences, GSK, Novartis, Roche; honoraria from AstraZeneca, GSK, Novartis, Thermo Fisher Scientific; travel accommodation and expenses from AstraZeneca, GSK, LCM Genect, Novartis, Roche, Thermo Fisher Scientific; grants and non-financial support from Thermo Fisher Scientific, Roche. CT has received consulting fee and served on the advisory boards for: Myriad Genetics, MSD Oncology and Amgen; honoraria for speakers’ bureaus from: Amgen; support for travel, accommodations, expenses from: Takeda, Amgen, MSD, Eli Lilly Italia SPA, Roche, Pfizer. AZ has received personal fees and non-financial support from Novartis, AstraZeneca, Lilly, Pfizer, Daiichi Sankyo, MDS (Merck Sharp&Dome), Roche, Seagen, Exact Sciences, Gilaed, Istituto Gentili. GP has served on the advisory boards for: ADS Biotec; has received honoraria for speakers’ bureaus and travel funding from: Lilly, AstraZeneca, Exact Sciences, Novartis. GB has received consulting fee from Roche, AstraZeneca, Novartis, MSD, Sanofi, Daiichi Sankyo, and Exact Sciences; honoraria for speakers’ bureaus from Roche, Pfizer, Astra- Zeneca, Lilly, Novartis, Neopharm Israel, MSD, Chugai, Daiichi Sankyo, EISAI, and Exact Sciences; support for travel, accommodations, expenses from Roche, Pfizer, and AstraZeneca; is co-inventor of ‘European patent Application N. 12195182.6 and 12196177.5 titled “PDL-1 expression in anti-HER2 therapy” -Roche- Issued (no compensation provided); and has served on the advisory boards for Roche, Pfizer, Daiichi Sankyo, Lilly, MSD, Novartis, AstraZeneca, Genomic Health, EISAI, Gilead, and Seagen.
Figures

Similar articles
-
Relationship of Oncotype Dx score with tumor grade, size, nodal status, proliferative marker Ki67 and Nottingham Prognostic Index in early breast cancer tumors in Saudi Population.Ann Diagn Pathol. 2021 Apr;51:151674. doi: 10.1016/j.anndiagpath.2020.151674. Epub 2020 Nov 25. Ann Diagn Pathol. 2021. PMID: 33360027
-
Discordance of Oncotype DX scores in synchronous bilateral and unilateral multifocal breast cancers.Breast Cancer Res Treat. 2024 Jan;203(1):73-83. doi: 10.1007/s10549-023-07119-3. Epub 2023 Sep 26. Breast Cancer Res Treat. 2024. PMID: 37751078
-
Correlation of the Ki67 Working Group prognostic risk categories with the Oncotype DX Recurrence Score in early breast cancer.Cancer. 2022 Oct;128(20):3602-3609. doi: 10.1002/cncr.34426. Epub 2022 Aug 10. Cancer. 2022. PMID: 35947048 Free PMC article.
-
Current controversies in the use of Oncotype DX in early breast cancer.Cancer Treat Rev. 2025 Apr;135:102887. doi: 10.1016/j.ctrv.2025.102887. Epub 2025 Jan 16. Cancer Treat Rev. 2025. PMID: 40048856 Review.
-
Multigene assays in early breast cancer: Insights from recent phase 3 studies.Eur J Surg Oncol. 2020 Apr;46(4 Pt A):656-666. doi: 10.1016/j.ejso.2019.10.019. Epub 2019 Oct 15. Eur J Surg Oncol. 2020. PMID: 31706719 Review.
Cited by
-
Correlation between histopathological features and recurrence score according to menopausal status in HR+/HER2- breast cancer patients: a retrospective study.Explor Target Antitumor Ther. 2025 Jul 18;6:1002331. doi: 10.37349/etat.2025.1002331. eCollection 2025. Explor Target Antitumor Ther. 2025. PMID: 40717834 Free PMC article.
-
Prediction of the 70-gene signature (MammaPrint) high versus low risk by nomograms among axillary lymph node positive (LN+) and negative (LN-) Chinese breast cancer patients, a retrospective study.BMC Cancer. 2025 Jul 1;25(1):1128. doi: 10.1186/s12885-025-14507-z. BMC Cancer. 2025. PMID: 40597882 Free PMC article.
-
The Evolving Role of Genomic Testing in Early Breast Cancer: Implications for Diagnosis, Prognosis, and Therapy.Int J Mol Sci. 2024 May 24;25(11):5717. doi: 10.3390/ijms25115717. Int J Mol Sci. 2024. PMID: 38891906 Free PMC article. Review.
References
-
- Henry NL, Somerfield MR, Abramson VG, et al. Role of patient and disease factors in adjuvant systemic therapy decision making for early-stage, operable breast cancer: update of the ASCO Endorsement of the Cancer Care Ontario Guideline. J Clin Oncol. 2019;37:1965–1977. doi: 10.1200/JCO.19.00948. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

