Trial of Selective Early Treatment of Patent Ductus Arteriosus with Ibuprofen
- PMID: 38265644
- PMCID: PMC7615774
- DOI: 10.1056/NEJMoa2305582
Trial of Selective Early Treatment of Patent Ductus Arteriosus with Ibuprofen
Abstract
Background: The cyclooxygenase inhibitor ibuprofen may be used to treat patent ductus arteriosus (PDA) in preterm infants. Whether selective early treatment of large PDAs with ibuprofen would improve short-term outcomes is not known.
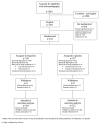
Methods: We conducted a multicenter, randomized, double-blind, placebo-controlled trial evaluating early treatment (≤72 hours after birth) with ibuprofen for a large PDA (diameter of ≥1.5 mm with pulsatile flow) in extremely preterm infants (born between 23 weeks 0 days' and 28 weeks 6 days' gestation). The primary outcome was a composite of death or moderate or severe bronchopulmonary dysplasia evaluated at 36 weeks of postmenstrual age.
Results: A total of 326 infants were assigned to receive ibuprofen and 327 to receive placebo; 324 and 322, respectively, had data available for outcome analyses. A primary-outcome event occurred in 220 of 318 infants (69.2%) in the ibuprofen group and 202 of 318 infants (63.5%) in the placebo group (adjusted risk ratio, 1.09; 95% confidence interval [CI], 0.98 to 1.20; P = 0.10). A total of 44 of 323 infants (13.6%) in the ibuprofen group and 33 of 321 infants (10.3%) in the placebo group died (adjusted risk ratio, 1.32; 95% CI, 0.92 to 1.90). Among the infants who survived to 36 weeks of postmenstrual age, moderate or severe bronchopulmonary dysplasia occurred in 176 of 274 (64.2%) in the ibuprofen group and 169 of 285 (59.3%) in the placebo group (adjusted risk ratio, 1.09; 95% CI, 0.96 to 1.23). Two unforeseeable serious adverse events occurred that were possibly related to ibuprofen.
Conclusions: The risk of death or moderate or severe bronchopulmonary dysplasia at 36 weeks of postmenstrual age was not significantly lower among infants who received early treatment with ibuprofen than among those who received placebo. (Funded by the National Institute for Health Research Health Technology Assessment Programme; Baby-OSCAR ISRCTN Registry number, ISRCTN84264977.).
Copyright © 2024 Massachusetts Medical Society.
Figures

Comment in
-
Early Treatment of Patent Ductus Arteriosus with Ibuprofen.N Engl J Med. 2024 Apr 4;390(13):1246. doi: 10.1056/NEJMc2402383. N Engl J Med. 2024. PMID: 38598589 No abstract available.
-
Early Treatment of Patent Ductus Arteriosus with Ibuprofen.N Engl J Med. 2024 Apr 4;390(13):1246-1247. doi: 10.1056/NEJMc2402383. N Engl J Med. 2024. PMID: 38598590 No abstract available.
-
Early Treatment of Patent Ductus Arteriosus with Ibuprofen. Reply.N Engl J Med. 2024 Apr 4;390(13):1247. doi: 10.1056/NEJMc2402383. N Engl J Med. 2024. PMID: 38598591 No abstract available.
References
-
- Sellmer A, Bjerre JV, Schmidt MR, et al. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. 2013;98:F505–10. - PubMed
-
- Semberova J, Sirc J, Miletin J, et al. Spontaneous Closure of Patent Ductus Arteriosus in Infants ≤1500 g. Pediatrics. 2017;140:e20164258. - PubMed
-
- Nemerofsky SL, Parravicini E, Bateman D, Kleinman C, Polin RA, Lorenz JM. The ductus arteriosus rarely requires treatment in infants > 1000 grams. Am J Perinatol. 2008;25:661–6. - PubMed
-
- Mirza H, Garcia J, McKinley G, et al. Duration of significant patent ductus arteriosus and bronchopulmonary dysplasia in extremely preterm infants. J Perinatol. 2019;39:1648–1655. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
