Sustained Remission Without Corticosteroids Among Patients With Pemphigus Who Had Rituximab as First-Line Therapy: Follow-Up of the Ritux 3 Trial
- PMID: 38265821
- PMCID: PMC10809134
- DOI: 10.1001/jamadermatol.2023.5679
Sustained Remission Without Corticosteroids Among Patients With Pemphigus Who Had Rituximab as First-Line Therapy: Follow-Up of the Ritux 3 Trial
Abstract
Importance: The Ritux 3 trial demonstrated the short-term efficacy and safety of first-line treatment with rituximab compared with a standard corticosteroid regimen in pemphigus. No data on the long-term follow-up of patients who received rituximab as first line are available.
Objective: To assess the long-term efficacy and safety of the Ritux 3 treatment regimen.
Design, setting, and participants: This 7-year follow-up study of the Ritux 3 trial included patients with pemphigus from 25 dermatology departments in France from January 1, 2010, to December 31, 2015.
Exposure: Patients were initially randomized in the rituximab plus prednisone group or prednisone-alone group.
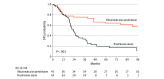
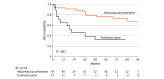
Main outcomes and measures: The primary outcome was the 5- and 7-year disease-free survival (DFS) without corticosteroids, assessed by Kaplan-Meier curves. Secondary outcomes were occurrence of relapse, occurrence of severe adverse events (SAEs), and evolution of antidesmoglein (Dsg) antibody enzyme-linked immunosorbent assay values to predict long-term relapse.
Results: Of the 90 patients in the Ritux 3 trial, 83 were evaluated at the end of follow-up study visit (44 in the rituximab plus prednisone group; 39 in the prednisone-alone group) with a median (IQR) follow-up of 87.3 (79.1-97.5) months. Forty-three patients (93%) from the rituximab plus prednisone and 17 patients (39%) from the prednisone-alone group had achieved complete remission without corticosteroids at any time during the follow-up. Patients from the rituximab group had much longer 5- and 7-year DFS without corticosteroids than patients from the prednisone-alone group (76.7% and 72.1% vs 35.3% and 35.3%, respectively; P < .001), and had about half the relapses (42.2% vs 83.7%; P < .001). Patients who received rituximab as second-line treatment had shorter DFS than patients treated as first line (P = .007). Fewer SAEs were reported in the rituximab plus prednisone group compared with the prednisone-alone group, 31 vs 58 respectively, corresponding to 0.67 and 1.32 SAEs per patient, respectively (P = .003). The combination of anti-Dsg1 values of 20 or more IU/mL and/or anti-Dsg3 values of 48 or more IU/mL yielded 0.83 positive predictive value and 0.94 negative predictive value to predict long-term relapse.
Conclusions and relevance: In this secondary analysis of the Ritux 3 trail, first-line treatment of patients with pemphigus with the Ritux 3 regimen was associated with long-term sustained complete remission without corticosteroid therapy without any additional maintenance infusion of rituximab.
Conflict of interest statement
Figures
References
-
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. ; French study group on autoimmune bullous skin diseases . First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389(10083):2031-2040. doi:10.1016/S0140-6736(17)30070-3 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

