Engineering an Autonucleolytic Mammalian Suspension Host Cell Line to Reduce DNA Impurity Levels in Serum-Free Lentiviral Process Streams
- PMID: 38266181
- PMCID: PMC10877604
- DOI: 10.1021/acssynbio.3c00682
Engineering an Autonucleolytic Mammalian Suspension Host Cell Line to Reduce DNA Impurity Levels in Serum-Free Lentiviral Process Streams
Abstract
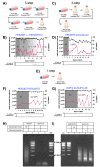
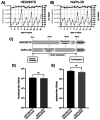
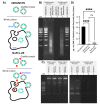
We engineered HEK293T cells with a transgene encoding tetracycline-inducible expression of a Staphylococcus aureus nuclease incorporating a translocation signal. We adapted the unmodified and nuclease-engineered cell lines to grow in suspension in serum-free media, generating the HEK293TS and NuPro-2S cell lines, respectively. Transient transfection yielded 1.19 × 106 lentiviral transducing units per milliliter (TU/mL) from NuPro-2S cells and 1.45 × 106 TU/mL from HEK293TS cells. DNA ladder disappearance revealed medium-resident nuclease activity arising from NuPro-2S cells in a tetracycline-inducible manner. DNA impurity levels in lentiviral material arising from NuPro-2S and HEK293TS cells were undetectable by SYBR Safe agarose gel staining. Direct measurement by PicoGreen reagent revealed DNA to be present at 636 ng/mL in lentiviral material from HEK293TS cells, an impurity level reduced by 89% to 70 ng/mL in lentiviral material from NuPro-2S cells. This reduction was comparable to the 23 ng/mL achieved by treating HEK293TS-derived lentiviral material with 50 units/mL Benzonase.
Keywords: bioprocess; gene therapy; lentivirus; mammalian cells; nuclease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Serum-free lentiviral vector production is compatible with medium-resident nuclease activity arising from adherent HEK293T host cells engineered with a nuclease-encoding transgene.Heliyon. 2023 Jun 7;9(6):e17067. doi: 10.1016/j.heliyon.2023.e17067. eCollection 2023 Jun. Heliyon. 2023. PMID: 37484388 Free PMC article.
-
An autonucleolytic suspension HEK293F host cell line for high-titer serum-free AAV5 and AAV9 production with reduced levels of DNA impurity.Mol Ther Methods Clin Dev. 2024 Aug 12;32(3):101317. doi: 10.1016/j.omtm.2024.101317. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39257529 Free PMC article.
-
Inducible packaging cells for large-scale production of lentiviral vectors in serum-free suspension culture.Mol Ther. 2008 Mar;16(3):500-7. doi: 10.1038/sj.mt.6300383. Epub 2008 Jan 8. Mol Ther. 2008. PMID: 18180776
-
Large-scale production means for the manufacturing of lentiviral vectors.Curr Gene Ther. 2010 Dec;10(6):474-86. doi: 10.2174/156652310793797748. Curr Gene Ther. 2010. PMID: 21054245 Review.
-
Lentiviral vectors: are they the future of animal transgenesis?Physiol Genomics. 2007 Oct 22;31(2):159-73. doi: 10.1152/physiolgenomics.00069.2007. Epub 2007 Aug 7. Physiol Genomics. 2007. PMID: 17684037 Review.
Cited by
-
Degradation of specific glycosaminoglycans improves transfection efficiency and vector production in transient lentiviral vector manufacturing processes.Front Bioeng Biotechnol. 2024 Jun 26;12:1409203. doi: 10.3389/fbioe.2024.1409203. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38994127 Free PMC article.
References
-
- July 24, 2020 Approval Letter - TECARTUS. U.S. Food and Drug Administration, July 24, 2020. https://www.fda.gov/media/140415/download (accessed 2022-12-21).
-
- August 30, 2017 Approval Letter - KYMRIAH. U.S. Food and Drug Administration, August 30, 2017. https://www.fda.gov/media/106989/download (accessed 2022-12-21).
-
- October 18, 2017 Approval Letter - YESCARTA. U.S. Food and Drug Administration, October 18, 2017. https://www.fda.gov/media/108458/download (accessed 2022-12-21).
-
- August 17, 2022 Approval Letter - ZYNTEGLO. U.S. Food and Drug Administration, August 17, 2022. https://www.fda.gov/media/160994/download (accessed 2022-12-21)
-
- September 16, 2022 Approval Letter - SKYSONA. U.S. Food and Drug Administration, September 16, 2022. https://www.fda.gov/media/161665/download (accessed 2022-12-21).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

