Mechanically Planar-to-Point Chirality Transmission in [2]Rotaxanes
- PMID: 38266249
- PMCID: PMC10859924
- DOI: 10.1021/jacs.3c11611
Mechanically Planar-to-Point Chirality Transmission in [2]Rotaxanes
Abstract
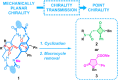
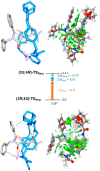
Herein we describe an effective transmission of chirality, from mechanically planar chirality to point chirality, in hydrogen-bonded [2]rotaxanes. A highly selective mono-N-methylation of one (out of four) amide N atom at the macrocyclic counterpart of starting achiral rotaxanes generates mechanically planar chirality. Followed by chiral resolution, both enantiomers were subjected to a base-promoted intramolecular cyclization, where their interlocked threads were transformed into new lactam moieties. As a matter of fact, the mechanically planar chiral information was effectively transferred to the resulting stereocenters (covalent chirality) of the newly formed heterocycles. Upon removing the entwined macrocycle, the final lactams were obtained with high enantiopurity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Jamieson E. M. G.; Modicom F.; Goldup S. M. Chirality in rotaxanes and catenanes. Chem. Soc. Rev. 2018, 47, 5266–5311. 10.1039/C8CS00097B. - DOI - PMC - PubMed
- Maynard J. R. J.; Goldup S. M. Strategies for the Synthesis of Enantiopure Mechanically Chiral Molecules. Chem 2020, 6, 1914–1932. 10.1016/j.chempr.2020.07.012. - DOI
-
- Martinez-Cuezva A.; Saura-Sanmartin A.; Alajarin M.; Berna J. Mechanically Interlocked Catalysts for Asymmetric Synthesis. ACS Catal. 2020, 10, 7719–7733. 10.1021/acscatal.0c02032. - DOI
-
- Lim J. Y. C.; Marques I.; Felix V.; Beer P. D. Enantioselective Anion Recognition by Chiral Halogen-Bonding [2]Rotaxanes. J. Am. Chem. Soc. 2017, 139, 12228–12239. 10.1021/jacs.7b06144. - DOI - PubMed
- Pairault N.; Niemeyer J. Chiral Mechanically Interlocked Molecules - Applications of Rotaxanes, Catenanes and Molecular Knots in Stereoselective Chemosensing and Catalysis. Synlett 2018, 29, 689–698. 10.1055/s-0036-1591934. - DOI
-
- Pezzato C.; Cheng C.; Stoddart J. F.; Astumian R. D. Mastering the non-equilibrium assembly and operation of molecular machines. Chem. Soc. Rev. 2017, 46, 5491–5507. 10.1039/C7CS00068E. - DOI - PubMed
- Mena-Hernando S.; Pérez E. M. Mechanically interlocked materials. Rotaxanes and catenanes beyond the small molecule. Chem. Soc. Rev. 2019, 48, 5016–5032. 10.1039/C8CS00888D. - DOI - PubMed
- Goujon A.; Moulin E.; Fuks G.; Giuseppone N. [c2]Daisy Chain Rotaxanes as Molecular Muscles. CCS Chem. 2019, 1, 83–96. 10.31635/ccschem.019.20180023. - DOI
- Corra S.; Curcio M.; Baroncini M.; Silvi S.; Credi A. Photoactivated Artificial Molecular Machines that Can Perform Tasks. Adv. Mater. 2020, 32, 1906064.10.1002/adma.201906064. - DOI - PubMed
- Zhou H.-Y.; Zong Q.-S.; Han Y.; Chen C.-F. Recent advances in higher order rotaxane architectures. Chem. Commun. 2020, 56, 9916–9936. 10.1039/D0CC03057K. - DOI - PubMed
- Chen L.; Sheng X.; Li G.; Huang F. Mechanically interlocked polymers based on rotaxanes. Chem. Soc. Rev. 2022, 51, 7046–7065. 10.1039/D2CS00202G. - DOI - PubMed
- Kato K.; Fa S.; Ohtani S.; Shi T. -h.; Brouwer A. M.; Ogoshi T. Noncovalently bound and mechanically interlocked systems using pillar[n]arenes. Chem. Soc. Rev. 2022, 51, 3648–3687. 10.1039/D2CS00169A. - DOI - PubMed
- Saura-Sanmartin A.; Pastor A.; Martinez-Cuezva A.; Cutillas-Font G.; Alajarin M.; Berna J. Mechanically interlocked molecules in metal–organic frameworks. Chem. Soc. Rev. 2022, 51, 4949–4976. 10.1039/D2CS00167E. - DOI - PubMed
-
- Bruns C. J.; Stoddart J. F.. The Nature of the Mechanical Bond: From Molecules to Machines; Wiley-VCH Verlag, 2016.
LinkOut - more resources
Full Text Sources
Research Materials

