Endothelial H2S-AMPK dysfunction upregulates the angiocrine factor PAI-1 and contributes to lung fibrosis
- PMID: 38266576
- PMCID: PMC10811458
- DOI: 10.1016/j.redox.2024.103038
Endothelial H2S-AMPK dysfunction upregulates the angiocrine factor PAI-1 and contributes to lung fibrosis
Abstract
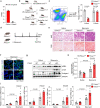
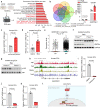
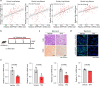
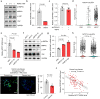
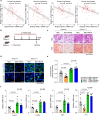
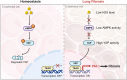
Dysfunction of the vascular angiocrine system is critically involved in regenerative defects and fibrosis of injured organs. Previous studies have identified various angiocrine factors and found that risk factors such as aging and metabolic disorders can disturb the vascular angiocrine system in fibrotic organs. One existing key gap is what sense the fibrotic risk to modulate the vascular angiocrine system in organ fibrosis. Here, using human and mouse data, we discovered that the metabolic pathway hydrogen sulfide (H2S)-AMP-activated protein kinase (AMPK) is a sensor of fibrotic stress and serves as a key mechanism upregulating the angiocrine factor plasminogen activator inhibitor-1 (PAI-1) in endothelial cells to participate in lung fibrosis. Activation of the metabolic sensor AMPK was inhibited in endothelial cells of fibrotic lungs, and AMPK inactivation was correlated with enriched fibrotic signature and reduced lung functions in humans. The inactivation of endothelial AMPK accelerated lung fibrosis in mice, while the activation of endothelial AMPK with metformin alleviated lung fibrosis. In fibrotic lungs, endothelial AMPK inactivation led to YAP activation and overexpression of the angiocrine factor PAI-1, which was positively correlated with the fibrotic signature in human fibrotic lungs and inhibition of PAI-1 with Tiplaxtinin mitigated lung fibrosis. Further study identified that the deficiency of the antioxidative gas metabolite H2S accounted for the inactivation of AMPK and activation of YAP-PAI-1 signaling in endothelial cells of fibrotic lungs. H2S deficiency was involved in human lung fibrosis and H2S supplement reversed mouse lung fibrosis in an endothelial AMPK-dependent manner. These findings provide new insight into the mechanism underlying the deregulation of the vascular angiocrine system in fibrotic organs.
Keywords: AMPK; H(2)S; Lung fibrosis; PAI-1; Vascular endothelial cells; YAP.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflict of interest.
Figures

References
-
- Zhao X., Kwan J.Y.Y., Yip K., Liu P.P., Liu F.F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 2020;19:57–75. - PubMed
-
- Koudstaal T., Funke-Chambour M., Kreuter M., Molyneaux P.L., Wijsenbeek M.S. Pulmonary fibrosis: from pathogenesis to clinical decision-making. Trends Mol. Med. 2023;29:1076–1087. - PubMed
-
- Liu G.Y., Budinger G.R.S., Dematte J.E. Advances in the management of idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. BMJ. 2022;377 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

