Prenatal arsenite exposure alters maternal cardiac remodeling during late pregnancy
- PMID: 38266874
- PMCID: PMC10922692
- DOI: 10.1016/j.taap.2024.116833
Prenatal arsenite exposure alters maternal cardiac remodeling during late pregnancy
Abstract
Exposure to inorganic arsenic through drinking water is widespread and has been linked to many chronic diseases, including cardiovascular disease. Arsenic exposure has been shown to alter hypertrophic signaling in the adult heart, as well as in utero offspring development. However, the effect of arsenic on maternal cardiac remodeling during pregnancy has not been studied. As such, there is a need to understand how environmental exposure contributes to adverse pregnancy-related cardiovascular events. This study seeks to understand the impact of trivalent inorganic arsenic exposure during gestation on maternal cardiac remodeling in late pregnancy, as well as offspring outcomes. C57BL/6 J mice were exposed to 0 (control), 100 or 1000 μg/L sodium arsenite (NaAsO2) beginning at embryonic day (E) 2.5 and continuing through E17.5. Maternal heart function and size were assessed via transthoracic echocardiography, gravimetric measurement, and histology. Transcript levels of hypertrophic markers were probed via qRT-PCR and confirmed by western blot. Offspring outcomes were assessed through echocardiography and gravimetric measurement. We found that maternal heart size was smaller and transcript levels of Esr1 (estrogen receptor alpha), Pgrmc1 (progesterone receptor membrane component 1) and Pgrmc2 (progesterone receptor membrane component 2) reduced during late pregnancy with exposure to 1000 μg/L iAs vs. non-exposed pregnant controls. Both 100 and 1000 μg/L iAs also reduced transcription of Nppa (atrial natriuretic peptide). Akt protein expression was also significantly reduced after 1000 μg/L iAs exposure in the maternal heart with no change in activating phosphorylation. This significant abrogation of maternal cardiac hypertrophy suggests that arsenic exposure during pregnancy can potentially contribute to cardiovascular disease. Taken together, our findings further underscore the importance of reducing arsenic exposure during pregnancy and indicate that more research is needed to assess the impact of arsenic and other environmental exposures on the maternal heart and adverse pregnancy events.
Keywords: Arsenic; Cardiotoxicity; Cardiovascular disease; Maternal exposure; Pregnancy.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Mark Kohr reports financial support was provided by National Heart Lung and Blood Institute. Nicole Taube reports financial support was provided by National Institute of Environmental Health Sciences. Haley Garbus reports financial support was provided by National Heart Lung and Blood Institute. Mark Kohr reports a relationship with National Heart Lung and Blood Institute that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





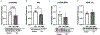

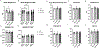
Update of
-
Prenatal Arsenite Exposure Alters Maternal Cardiac Remodeling During Late Pregnancy.bioRxiv [Preprint]. 2023 Sep 29:2023.09.28.559986. doi: 10.1101/2023.09.28.559986. bioRxiv. 2023. Update in: Toxicol Appl Pharmacol. 2024 Feb;483:116833. doi: 10.1016/j.taap.2024.116833. PMID: 37808684 Free PMC article. Updated. Preprint.
References
-
- Substance Priority List | ATSDR. https://www.atsdr.cdc.gov/spl/index.html.
-
- Nordstrom DK PUBLIC HEALTH: Enhanced: Worldwide Occurrences of Arsenic in Ground Water. Science (1979) 296, 2143–2145 (2002). - PubMed
-
- Abernathy CO, Thomas DJ & Calderon RL Toxicity and Risk Assessment of Trace Elements Health Effects and Risk Assessment of Arsenic 1,2.
-
- Vahter M Mechanisms of arsenic biotransformation. Toxicology 181–182, 211–217 (2002). - PubMed
-
- Ferreccio C et al. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology 11, 673–679 (2000). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

