Establishment of an organ culture system to maintain the structure of mouse Müllerian ducts during development
- PMID: 38267037
- PMCID: PMC10963091
- DOI: 10.1292/jvms.23-0492
Establishment of an organ culture system to maintain the structure of mouse Müllerian ducts during development
Abstract
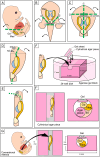
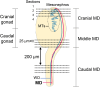
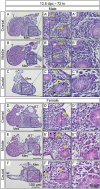
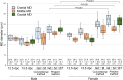
We previously showed that the anti-Müllerian hormone (AMH), infiltrating from the testis to the mesonephros reaches the cranial and middle regions of the Müllerian duct (MD) and induces their regression using an organ culture in mice. However, it is difficult to maintain structural integrity, such as the length and diameter and normal direction of elongation of the caudal region of the MD, in conventional organ culture systems. Therefore, the pathway of AMH to the caudal MD region remains uncharted. In this study, we established an organ culture method that can maintain the morphology of the caudal region of the MD. The gonad-mesonephros complex, metanephros, and urinary bladder of mouse fetuses at 12.5 dpc attached to the body trunk were cultured on agarose gels for 72 hr. The cultured caudal region of the mesonephros was elongated along the body trunk, and the course of the mesonephros was maintained in many individuals. In males, mesenchymal cells aggregated around the MD after culture. Moreover, the male MD diameter was significantly smaller than the female. Based on these results, it was concluded that the development of the MD was maintained in the present organ culture system. Using this culture system, AMH infiltration to the caudal region of the MD can be examined without the influence of AMH in the blood. This culture system is useful for clarifying the regression mechanism of the caudal region of the MD.
Keywords: Müllerian duct regression; anti-Müllerian hormone (AMH); organ culture; secretion manner; sexual differentiation.
Conflict of interest statement
The authors have no conflicts of interest.
Figures


Similar articles
-
Direct diffusion of anti-Müllerian hormone from both the cranial and caudal regions of the testis during early gonadal development in mice.Dev Dyn. 2024 Mar;253(3):296-311. doi: 10.1002/dvdy.662. Epub 2023 Oct 3. Dev Dyn. 2024. PMID: 37787412
-
The mechanisms underlying the effects of AMH on Müllerian duct regression in male mice.J Vet Med Sci. 2018 Apr 18;80(4):557-567. doi: 10.1292/jvms.18-0023. Epub 2018 Mar 9. J Vet Med Sci. 2018. PMID: 29526868 Free PMC article.
-
Establishment of an organ culture system to induce Sertoli cell differentiation from undifferentiated mouse gonads.J Vet Med Sci. 2020 Apr 9;82(4):414-421. doi: 10.1292/jvms.20-0036. Epub 2020 Feb 21. J Vet Med Sci. 2020. PMID: 32092744 Free PMC article.
-
Anti-Müllerian hormone: A function beyond the Müllerian structures.Morphologie. 2022 Dec;106(355):252-259. doi: 10.1016/j.morpho.2021.11.002. Epub 2021 Dec 16. Morphologie. 2022. PMID: 34924282 Review.
-
The in vivo roles of müllerian-inhibiting substance.Curr Top Dev Biol. 1994;29:171-87. doi: 10.1016/s0070-2153(08)60550-5. Curr Top Dev Biol. 1994. PMID: 7828438 Review.
References
-
- Bentvelsen FM, Brinkmann AO, van der Schoot P, van der Linden JE, van der Kwast TH, Boersma WJ, Schröder FH, Nijman JM. 1995. Developmental pattern and regulation by androgens of androgen receptor expression in the urogenital tract of the rat. Mol Cell Endocrinol 113: 245–253. doi: 10.1016/0303-7207(95)03593-V - DOI - PubMed

