Diurnal Rhythms in the Red Seaweed Gracilariopsis chorda are Characterized by Unique Regulatory Networks of Carbon Metabolism
- PMID: 38267085
- PMCID: PMC10853006
- DOI: 10.1093/molbev/msae012
Diurnal Rhythms in the Red Seaweed Gracilariopsis chorda are Characterized by Unique Regulatory Networks of Carbon Metabolism
Abstract
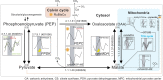
Cellular and physiological cycles are driven by endogenous pacemakers, the diurnal and circadian rhythms. Key functions such as cell cycle progression and cellular metabolism are under rhythmic regulation, thereby maintaining physiological homeostasis. The photoreceptors phytochrome and cryptochrome, in response to light cues, are central input pathways for physiological cycles in most photosynthetic organisms. However, among Archaeplastida, red algae are the only taxa that lack phytochromes. Current knowledge about oscillatory rhythms is primarily derived from model species such as Arabidopsis thaliana and Chlamydomonas reinhardtii in the Viridiplantae, whereas little is known about these processes in other clades of the Archaeplastida, such as the red algae (Rhodophyta). We used genome-wide expression profiling of the red seaweed Gracilariopsis chorda and identified 3,098 rhythmic genes. Here, we characterized possible cryptochrome-based regulation and photosynthetic/cytosolic carbon metabolism in this species. We found a large family of cryptochrome genes in G. chorda that display rhythmic expression over the diurnal cycle and may compensate for the lack of phytochromes in this species. The input pathway gates regulatory networks of carbon metabolism which results in a compact and efficient energy metabolism during daylight hours. The system in G. chorda is distinct from energy metabolism in most plants, which activates in the dark. The green lineage, in particular, land plants, balance water loss and CO2 capture in terrestrial environments. In contrast, red seaweeds maintain a reduced set of photoreceptors and a compact cytosolic carbon metabolism to thrive in the harsh abiotic conditions typical of intertidal zones.
Keywords: Gracilariopsis chorda; cryptochrome; cytosolic carbon metabolism; horizontal gene transfers; rhythmic gene.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Conflict of interest statement
Conflict of Interest: The authors declare no competing interests.
Figures



Similar articles
-
From dusk till dawn: cell cycle progression in the red seaweed Gracilariopsis chorda (Rhodophyta).iScience. 2024 Jun 6;27(7):110190. doi: 10.1016/j.isci.2024.110190. eCollection 2024 Jul 19. iScience. 2024. PMID: 38984202 Free PMC article.
-
Analysis of the Draft Genome of the Red Seaweed Gracilariopsis chorda Provides Insights into Genome Size Evolution in Rhodophyta.Mol Biol Evol. 2018 Aug 1;35(8):1869-1886. doi: 10.1093/molbev/msy081. Mol Biol Evol. 2018. PMID: 29688518
-
Expression of seven carbonic anhydrases in red alga Gracilariopsis chorda and their subcellular localization in a heterologous system, Arabidopsis thaliana.Plant Cell Rep. 2019 Feb;38(2):147-159. doi: 10.1007/s00299-018-2356-8. Epub 2018 Nov 16. Plant Cell Rep. 2019. PMID: 30446790
-
Cryptochrome photoreceptors in green algae: Unexpected versatility of mechanisms and functions.J Plant Physiol. 2017 Oct;217:4-14. doi: 10.1016/j.jplph.2017.05.021. Epub 2017 May 31. J Plant Physiol. 2017. PMID: 28619534 Review.
-
The World of Algae Reveals a Broad Variety of Cryptochrome Properties and Functions.Front Plant Sci. 2021 Nov 1;12:766509. doi: 10.3389/fpls.2021.766509. eCollection 2021. Front Plant Sci. 2021. PMID: 34790217 Free PMC article. Review.
Cited by
-
From dusk till dawn: cell cycle progression in the red seaweed Gracilariopsis chorda (Rhodophyta).iScience. 2024 Jun 6;27(7):110190. doi: 10.1016/j.isci.2024.110190. eCollection 2024 Jul 19. iScience. 2024. PMID: 38984202 Free PMC article.
References
-
- Artus NN, Edwards GE. Properties of leaf NAD-malic enzyme from the inducible crassulacean acid metabolism species Mesembryanthemum crystallinum. Plant Cell Physiol. 1985:26(2):341–350. 10.1093/oxfordjournals.pcp.a076915 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

