HCV Antiviral Drugs Have the Potential to Adversely Perturb the Fetal-Maternal Communication Axis through Inhibition of CYP3A7 DHEA-S Oxidation
- PMID: 38267095
- PMCID: PMC11114604
- DOI: 10.1124/dmd.123.001434
HCV Antiviral Drugs Have the Potential to Adversely Perturb the Fetal-Maternal Communication Axis through Inhibition of CYP3A7 DHEA-S Oxidation
Abstract
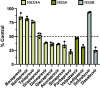
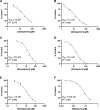
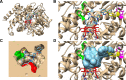
The hepatitis C virus (HCV) poses a great risk to pregnant people and their developing fetus, yet no HCV antiviral treatment guidelines have been established. While there has been a substantial increase in the development of HCV antivirals, the effect they have on the developing fetus remains poorly defined. Many of these drugs are metabolized through the cytochrome P450 CYP3A pathway, which is mediated by cytochrome P450 3A7 (CYP3A7) in the fetus and developing infant. In this study, we sought to investigate the effect HCV antivirals have on CYP3A7 metabolism, as this CYP enzyme plays a vital role in proper fetal and neonatal development. Of the 13 HCV antivirals we investigated, 8 (∼62%) inhibited CYP3A7 metabolic activity by 50% or more at a concentration of 20 µM. Furthermore, paritaprevir, asunaprevir, simeprevir, danoprevir, and glecaprevir all had observed half-maximal inhibitory concentrations between the range of 10 and 20 µM, which is physiologically relevant in comparison with the Km of dehydroepiandrosterone-sulfate (DHEA-S) oxidation (reported to be between 5 and 20 µM). We also discovered that paritaprevir is a time-dependent inhibitor of CYP3A7, which shifts the IC50 ∼twofold from 11 µM to 5 µM. Upon further characterization, paritaprevir inactivates DHEA-S metabolism by CYP3A7, with KI and Kinact values of 4.66 µM and 0.00954 minute-1, respectively. Depending on treatment plan and off-label drug use, HCV treatment could adversely affect the fetal-maternal communication axis by blocking fetal CYP3A7 metabolism of important endogenous hormones. SIGNIFICANCE STATEMENT: The prevalence of HCV in pregnant people is estimated at between 1% and 8% of the global population, yet little to no information exists about the risk antiviral treatment poses to the developing fetus. There is a potential risk of drugs adversely affecting mother-fetal communication by inhibiting fetal hepatic CYP3A7, an integral enzyme for estriol production. We discovered that five HCV antivirals inhibited DHEA-S metabolism by CYP3A7, and paritaprevir inactivated the enzyme. Our studies demonstrate the potential threat these drugs pose to proper fetal development.
Copyright © 2024 by The Author(s).
Figures


Similar articles
-
Inhibition of CYP3A7 DHEA-S Oxidation by Lopinavir and Ritonavir: An Alternative Mechanism for Adrenal Impairment in HIV Antiretroviral-Treated Neonates.Chem Res Toxicol. 2021 Apr 19;34(4):1150-1160. doi: 10.1021/acs.chemrestox.1c00028. Epub 2021 Apr 6. Chem Res Toxicol. 2021. PMID: 33821626 Free PMC article.
-
Comparison of the CYP3A Selective Inhibitors CYP3cide, Clobetasol, and Azamulin for Their Potential to Distinguish CYP3A7 Activity in the Presence of CYP3A4/5.Drug Metab Dispos. 2024 Oct 16;52(11):1224-1233. doi: 10.1124/dmd.124.001598. Drug Metab Dispos. 2024. PMID: 38702193 Free PMC article.
-
Neonatal cytochrome P450 CYP3A7: A comprehensive review of its role in development, disease, and xenobiotic metabolism.Arch Biochem Biophys. 2019 Sep 30;673:108078. doi: 10.1016/j.abb.2019.108078. Epub 2019 Aug 22. Arch Biochem Biophys. 2019. PMID: 31445893 Free PMC article. Review.
-
Human cytochrome P450 3A7 binding four copies of its native substrate dehydroepiandrosterone 3-sulfate.J Biol Chem. 2023 Aug;299(8):104993. doi: 10.1016/j.jbc.2023.104993. Epub 2023 Jun 29. J Biol Chem. 2023. PMID: 37392852 Free PMC article.
-
Genetic contribution to variable human CYP3A-mediated metabolism.Adv Drug Deliv Rev. 2002 Nov 18;54(10):1271-94. doi: 10.1016/s0169-409x(02)00066-2. Adv Drug Deliv Rev. 2002. PMID: 12406645 Review.
References
-
- Blach STerrault NATacke FGamkrelidze ICraxi ATanaka JWaked IDore GJAbbas ZAbdallah AR, et al. ; Polaris Observatory HCV Collaborators (2022) Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol 7:396–415 DOI: 10.1016/S2468-1253(21)00472-6. - DOI - PubMed
-
- Brennan BJ, Poirier A, Moreira S, Morcos PN, Goelzer P, Portmann R, Asthappan J, Funk C, Smith PF (2015) Characterization of the transmembrane transport and absolute bioavailability of the HCV protease inhibitor danoprevir. Clin Pharmacokinet 54:537–549 DOI: 10.1007/s40262-014-0222-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

