Time-restricted eating and supervised exercise for improving hepatic steatosis and cardiometabolic health in adults with obesity: protocol for the TEMPUS randomised controlled trial
- PMID: 38267239
- PMCID: PMC10824004
- DOI: 10.1136/bmjopen-2023-078472
Time-restricted eating and supervised exercise for improving hepatic steatosis and cardiometabolic health in adults with obesity: protocol for the TEMPUS randomised controlled trial
Abstract
Introduction: Metabolic dysfunction-associated steatotic liver disease is a major public health problem considering its high prevalence and its strong association with extrahepatic diseases. Implementing strategies based on an intermittent fasting approach and supervised exercise may mitigate the risks. This study aims to investigate the effects of a 12-week time-restricted eating (TRE) intervention combined with a supervised exercise intervention, compared with TRE or supervised exercise alone and with a usual-care control group, on hepatic fat (primary outcome) and cardiometabolic health (secondary outcomes) in adults with obesity.
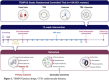
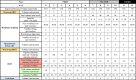
Methods and analysis: An anticipated 184 adults with obesity (50% women) will be recruited from Granada (south of Spain) for this parallel-group, randomised controlled trial (TEMPUS). Participants will be randomly designated to usual care, TRE alone, supervised exercise alone or TRE combined with supervised exercise, using a parallel design with a 1:1:1:1 allocation ratio. The TRE and TRE combined with supervised exercise groups will select an 8-hour eating window before the intervention and will maintain it over the intervention. The exercise alone and TRE combined with exercise groups will perform 24 sessions (2 sessions per week+walking intervention) of supervised exercise combining resistance and aerobic high-intensity interval training. All participants will receive nutritional counselling throughout the intervention. The primary outcome is change from baseline to 12 weeks in hepatic fat; secondary outcomes include measures of cardiometabolic health.
Ethics and dissemination: This study was approved by Granada Provincial Research Ethics Committee (CEI Granada-0365-N-23). All participants will be asked to provide written informed consent. The findings will be disseminated in scientific journals and at international scientific conferences.
Trial registration number: NCT05897073.
Keywords: Cardiovascular imaging; General endocrinology; Obesity.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- WHO Regional office for Europe . WHO European Regional Obesity Report 2022. 2022: 1–220.
-
- Lazarus JV, Newsome PN, Francque SM, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology November 20, 2023. 10.1097/HEP.0000000000000696 Available: http://www.journal-of-hepatology.eu/article/S016882782300418X/fulltext - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
