Treatment-related pneumonitis after thoracic radiotherapy/chemoradiotherapy combined with anti-PD-1 monoclonal antibodies in advanced esophageal squamous cell carcinoma
- PMID: 38267589
- PMCID: PMC11442583
- DOI: 10.1007/s00066-024-02199-6
Treatment-related pneumonitis after thoracic radiotherapy/chemoradiotherapy combined with anti-PD-1 monoclonal antibodies in advanced esophageal squamous cell carcinoma
Abstract
Purpose: This study aims to evaluate the risk factors of treatment-related pneumonitis (TRP) following thoracic radiotherapy/chemoradiotherapy combined with anti-PD‑1 monoclonal antibodies (mAbs) in patients with advanced esophageal squamous cell carcinoma (ESCC).
Methods: We retrospectively reviewed 97 patients with advanced ESCC who were treated with thoracic radiotherapy/chemoradiotherapy combined with anti-PD‑1 mAbs. Among them, 56 patients received concurrent radiotherapy with anti-PD‑1 mAbs and 41 patients received sequential radiotherapy with anti-PD‑1 mAbs. The median prescribed planning target volume (PTV) dose was 59.4 Gy (range from 50.4 to 66 Gy, 1.8-2.2 Gy/fraction). Clinical characteristics, the percentage of lung volume receiving more than 5-50 Gy in increments of 5 Gy (V5-V50, respectively) and the mean lung dose (MLD) were analyzed as potential risk factors for TRP.
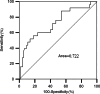
Results: 46.4% (45/97), 20.6% (20/97), 20.6% (20/97), 4.1% (4/97), and 1.0% (1/97) of the patients developed any grade of TRP, grade 1 TRP, grade 2 TRP, grade 3 TRP, and fatal (grade 5) TRP, respectively. Anti-PD‑1 mAbs administered concurrently with radiotherapy, V5, V10, V15, V25, V30, V35, V40 and MLD were associated with the occurrence of grade 2 or higher TRP. Concurrent therapy (P = 0.010, OR = 3.990) and V5 (P = 0.001, OR = 1.126) were independent risk factors for grade 2 or higher TRP. According to the receiver operating characteristic (ROC) curve analysis, the optimal V5 threshold for predicting grade 2 or higher TRP was 55.7%.
Conclusion: The combination of thoracic radiotherapy/chemoradiotherapy with anti-PD‑1 mAbs displayed a tolerable pulmonary safety profile. Although the incidence of TRP was high, grade 1-2 TRP accounted for the majority. Anti-PD‑1 mAbs administered concurrently with radiotherapy and the lung V5 were significantly associated with the occurrence of grade 2 or higher TRP. Therefore, it seems safer to control V5 below 55% in clinical, especially for the high-risk populations receiving concurrent therapy.
Keywords: Anti-PD‑1 monoclonal antibodies; Dosimetric parameters; Esophageal squamous cell carcinoma; Pneumonitis; Thoracic radiotherapy.
© 2024. The Author(s).
Conflict of interest statement
X. Lv, Y. Wu, Q. Li, C. Zheng, Q. Lin, Q. Pang, M. Zhao, J. Zhang and J. Wang declare that they have no competing interests.
Figures
Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Impact of first-line chemoimmunotherapy with or without radiotherapy on the prognosis of patients with locally advanced or metastatic esophageal squamous cell carcinoma: a multicenter, real-world, retrospective cohort study from China (NCT06478355).Front Immunol. 2025 Jul 28;16:1633930. doi: 10.3389/fimmu.2025.1633930. eCollection 2025. Front Immunol. 2025. PMID: 40791599 Free PMC article. Clinical Trial.
-
Case report: Long-term survival in synchronous double primary malignancies of lung adenocarcinomas and esophageal squamous cell carcinoma treated with definitive chemoradiotherapy and SBRT combined with anti-PD-1.Front Immunol. 2025 Feb 14;16:1548176. doi: 10.3389/fimmu.2025.1548176. eCollection 2025. Front Immunol. 2025. PMID: 40028319 Free PMC article.
-
Evaluation of Radiation Pneumonitis in a Phase 2 Study of Consolidation Immunotherapy With Nivolumab and Ipilimumab or Nivolumab Alone Following Concurrent Chemoradiation Therapy for Unresectable Stage IIIA/IIIB Non-Small Cell Lung Cancer.Int J Radiat Oncol Biol Phys. 2025 Mar 1;121(3):720-727. doi: 10.1016/j.ijrobp.2024.09.050. Epub 2024 Oct 26. Int J Radiat Oncol Biol Phys. 2025. PMID: 39490906 Clinical Trial.
-
Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis.Oncologist. 2017 Apr;22(4):470-479. doi: 10.1634/theoncologist.2016-0419. Epub 2017 Mar 8. Oncologist. 2017. PMID: 28275115 Free PMC article.
Cited by
-
A Systematic Review of Pneumonitis Following Treatment with Immune Checkpoint Inhibitors and Radiotherapy.Biomedicines. 2025 Apr 12;13(4):946. doi: 10.3390/biomedicines13040946. Biomedicines. 2025. PMID: 40299683 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185countries. CA Cancer J Clin 68:394–424. 10.3322/caac.21492 - PubMed
-
- Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH et al (2020) Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 38(35):4138–4148. 10.1200/JCO.20.01888 - PubMed
-
- Pelosof L, Saung MT, Donoghue M, Casak S, Mushti S, Cheng J et al (2021) Benefit-risk summary of nivolumab for the treatment of patients with unresectable advanced, recurrent, or metastatic esophageal squamous cell carcinoma after prior Fluoropyrimidine- and platinum-based chemotherapy. Oncologist 26(4):318–324. 10.1002/onco.13646 - PMC - PubMed
-
- Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z et al (2020) Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 21(6):832–842. 10.1016/S1470-2045(20)30110-8 - PubMed
-
- Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T et al (2021) Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398(10302):759–771. 10.1016/S0140-6736(21)01234-4 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical