Cortactin stabilizes actin branches by bridging activated Arp2/3 to its nucleated actin filament
- PMID: 38267598
- PMCID: PMC11102864
- DOI: 10.1038/s41594-023-01205-2
Cortactin stabilizes actin branches by bridging activated Arp2/3 to its nucleated actin filament
Abstract
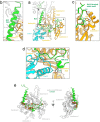
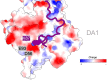
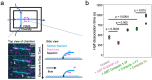
Regulation of the assembly and turnover of branched actin filament networks nucleated by the Arp2/3 complex is essential during many cellular processes, including cell migration and membrane trafficking. Cortactin is important for actin branch stabilization, but the mechanism by which this occurs is unclear. Given this, we determined the structure of vertebrate cortactin-stabilized Arp2/3 actin branches using cryogenic electron microscopy. We find that cortactin interacts with the new daughter filament nucleated by the Arp2/3 complex at the branch site, rather than the initial mother actin filament. Cortactin preferentially binds activated Arp3. It also stabilizes the F-actin-like interface of activated Arp3 with the first actin subunit of the new filament, and its central repeats extend along successive daughter-filament subunits. The preference of cortactin for activated Arp3 explains its retention at the actin branch and accounts for its synergy with other nucleation-promoting factors in regulating branched actin network dynamics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
Abl2/Abl-related gene stabilizes actin filaments, stimulates actin branching by actin-related protein 2/3 complex, and promotes actin filament severing by cofilin.J Biol Chem. 2015 Feb 13;290(7):4038-46. doi: 10.1074/jbc.M114.608117. Epub 2014 Dec 24. J Biol Chem. 2015. PMID: 25540195 Free PMC article.
-
Cortactin binding to F-actin revealed by electron microscopy and 3D reconstruction.J Mol Biol. 2006 Jun 16;359(4):840-7. doi: 10.1016/j.jmb.2006.03.065. Epub 2006 May 4. J Mol Biol. 2006. PMID: 16697006
-
Interactions with actin monomers, actin filaments, and Arp2/3 complex define the roles of WASP family proteins and cortactin in coordinately regulating branched actin networks.J Biol Chem. 2014 Oct 17;289(42):28856-69. doi: 10.1074/jbc.M114.587527. Epub 2014 Aug 26. J Biol Chem. 2014. PMID: 25160634 Free PMC article.
-
Nucleation, stabilization, and disassembly of branched actin networks.Trends Cell Biol. 2022 May;32(5):421-432. doi: 10.1016/j.tcb.2021.10.006. Epub 2021 Nov 23. Trends Cell Biol. 2022. PMID: 34836783 Free PMC article. Review.
-
The stabilization of Arp2/3 complex generated actin filaments.Biochem Soc Trans. 2024 Feb 28;52(1):343-352. doi: 10.1042/BST20230638. Biochem Soc Trans. 2024. PMID: 38288872 Free PMC article. Review.
Cited by
-
Two ligands of Arp2/3 complex, yeast coronin and GMF, interact and synergize in pruning branched actin networks.J Biol Chem. 2025 Mar;301(3):108191. doi: 10.1016/j.jbc.2025.108191. Epub 2025 Jan 16. J Biol Chem. 2025. PMID: 39826693 Free PMC article.
-
Regeneration of actin filament branches from the same Arp2/3 complex.Sci Adv. 2024 Jan 26;10(4):eadj7681. doi: 10.1126/sciadv.adj7681. Epub 2024 Jan 26. Sci Adv. 2024. PMID: 38277459 Free PMC article.
-
NPF binding to Arp2 is allosterically linked to the release of ArpC5's N-terminal tail and conformational changes in Arp2/3 complex.Proc Natl Acad Sci U S A. 2025 Feb 25;122(8):e2421557122. doi: 10.1073/pnas.2421557122. Epub 2025 Feb 18. Proc Natl Acad Sci U S A. 2025. PMID: 40042350 Free PMC article.
-
Cytoskeletal mechanisms regulating attaching/effacing bacteria interactions with host cells: It takes a village to build the pedestal.Bioessays. 2024 Nov;46(11):e2400160. doi: 10.1002/bies.202400160. Epub 2024 Sep 20. Bioessays. 2024. PMID: 39301984 Review.
-
Cell condensation initiates organogenesis: the role of actin dynamics in supracellular self-organizing process.Cell Biosci. 2025 Jul 13;15(1):101. doi: 10.1186/s13578-025-01429-3. Cell Biosci. 2025. PMID: 40653479 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

