In Vitro and In Vivo testing of 3D-Printed Amorphous Lopinavir Printlets by Selective Laser Sinitering: Improved Bioavailability of a Poorly Soluble Drug
- PMID: 38267637
- PMCID: PMC11698493
- DOI: 10.1208/s12249-023-02729-y
In Vitro and In Vivo testing of 3D-Printed Amorphous Lopinavir Printlets by Selective Laser Sinitering: Improved Bioavailability of a Poorly Soluble Drug
Abstract
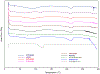
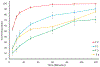
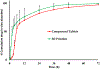
The aim of this paper was to investigate the effects of formulation parameters on the physicochemical and pharmacokinetic (PK) behavior of amorphous printlets of lopinavir (LPV) manufactured by selective laser sintering 3D printing method (SLS). The formulation variables investigated were disintegrants (magnesium aluminum silicate at 5-10%, microcrystalline cellulose at 10-20%) and the polymer (Kollicoat® IR at 42-57%), while keeping printing parameters constant. Differential scanning calorimetry, X-ray powder diffraction, and Fourier-transform infrared analysis confirmed the transformation of the crystalline drug into an amorphous form. A direct correlation was found between the disintegrant concentration and dissolution. The dissolved drug ranged from 71.1 ± 5.7% to 99.3 ± 2.7% within 120 min. A comparative PK study in rabbits showed significant differences in the rate and extent of absorption between printlets and compressed tablets. The values for Tmax, Cmax, and AUC were 4 times faster, and 2.5 and 1.7 times higher in the printlets compared to the compressed tablets, respectively. In conclusion, the SLS printing method can be used to create an amorphous delivery system through a single continuous process.
Keywords: amorphous solid dispersion; dissolution; lopinavir; pharmacokinetics; printlets; selective laser sintering.
© 2024. The Author(s), under exclusive licence to American Association of Pharmaceutical Scientists.
Conflict of interest statement
Figures



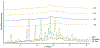

Similar articles
-
3D-printing of lopinavir printlets by selective laser sintering and quantification of crystalline fraction by XRPD-chemometric models.Int J Pharm. 2021 Jan 5;592:120059. doi: 10.1016/j.ijpharm.2020.120059. Epub 2020 Nov 7. Int J Pharm. 2021. PMID: 33171261
-
Development, Pharmacokinetics and Antimalarial Evaluation of Dose Flexible 3D Printlets of Dapsone for Pediatric Patients.AAPS PharmSciTech. 2024 Sep 17;25(7):217. doi: 10.1208/s12249-024-02935-2. AAPS PharmSciTech. 2024. PMID: 39289236 Free PMC article.
-
Very-Rapidly Dissolving Printlets of Isoniazid Manufactured by SLS 3D Printing: In Vitro and In Vivo Characterization.Mol Pharm. 2022 Aug 1;19(8):2937-2949. doi: 10.1021/acs.molpharmaceut.2c00306. Epub 2022 Jun 1. Mol Pharm. 2022. PMID: 35648147 Free PMC article.
-
3D printing: Principles and pharmaceutical applications of selective laser sintering.Int J Pharm. 2020 Aug 30;586:119594. doi: 10.1016/j.ijpharm.2020.119594. Epub 2020 Jul 2. Int J Pharm. 2020. PMID: 32622811 Review.
-
Selective laser sintering 3D printing - an overview of the technology and pharmaceutical applications.Drug Dev Ind Pharm. 2020 Jun;46(6):869-877. doi: 10.1080/03639045.2020.1764027. Epub 2020 May 13. Drug Dev Ind Pharm. 2020. PMID: 32364418 Review.
Cited by
-
Development and Characterization of Printlets of Lamivudine for Pediatric Patients Using Selective Laser Sintering.Pharmaceuticals (Basel). 2025 Mar 1;18(3):356. doi: 10.3390/ph18030356. Pharmaceuticals (Basel). 2025. PMID: 40143133 Free PMC article.
-
Twin Screw Melt Granulation of Simvastatin: Drug Solubility and Dissolution Rate Enhancement Using Polymer Blends.Pharmaceutics. 2024 Dec 23;16(12):1630. doi: 10.3390/pharmaceutics16121630. Pharmaceutics. 2024. PMID: 39771607 Free PMC article.
-
Cold Laser Sintering of Medicines: Toward Carbon Neutral Pharmaceutical Printing.ACS Sustain Chem Eng. 2024 Jul 16;12(30):11155-11166. doi: 10.1021/acssuschemeng.4c01439. eCollection 2024 Jul 29. ACS Sustain Chem Eng. 2024. PMID: 39091925 Free PMC article.
References
-
- Wu C-j, You J-z, Wang X-j. Thermal decomposition mechanism of tenofovir disoproxil fumarate. J Therm Anal Calorim. 2018;132(1):471–82. 10.1007/s10973-017-6910-3. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

