Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction
- PMID: 38267706
- PMCID: PMC10950785
- DOI: 10.1038/s43587-023-00560-5
Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction
Abstract
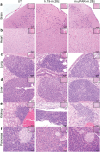
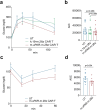
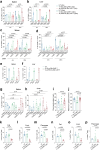
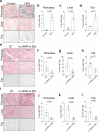
Senescent cells, which accumulate in organisms over time, contribute to age-related tissue decline. Genetic ablation of senescent cells can ameliorate various age-related pathologies, including metabolic dysfunction and decreased physical fitness. While small-molecule drugs that eliminate senescent cells ('senolytics') partially replicate these phenotypes, they require continuous administration. We have developed a senolytic therapy based on chimeric antigen receptor (CAR) T cells targeting the senescence-associated protein urokinase plasminogen activator receptor (uPAR), and we previously showed these can safely eliminate senescent cells in young animals. We now show that uPAR-positive senescent cells accumulate during aging and that they can be safely targeted with senolytic CAR T cells. Treatment with anti-uPAR CAR T cells improves exercise capacity in physiological aging, and it ameliorates metabolic dysfunction (for example, improving glucose tolerance) in aged mice and in mice on a high-fat diet. Importantly, a single administration of these senolytic CAR T cells is sufficient to achieve long-term therapeutic and preventive effects.
© 2024. The Author(s).
Conflict of interest statement
C.A., J.F., M.S. and S.W.L. are listed as the inventors of several patent applications (62/800,188; 63/174,277; 63/209,941; 63/209,940; 63/209,915; 63/209,924; 17/426,728; 3,128,368; 20748891.7; 2020216486) related to senolytic CAR T cells. M.S. holds other unrelated patents on CAR technologies. C.A. is also an inventor in the patent application 63/510,997. C.A., M.S. and S.W.L. are advisors for Fate Therapeutics. M.S. also receives research support from Fate Therapeutics, is an advisor and has equity in Senecea Therapeutics, and holds other unrelated patents on CAR technologies. S.W.L. is on the scientific advisory board and has equity in ORIC Pharmaceuticals, Blueprint Medicines, Mirimus, Senecea Therapeutics, Faeth Therapeutics and PMV Pharmaceuticals. D.A.T. is a scientific cofounder and scientific advisory board of Mestag Therapeutics and is a member of the Scientific Advisory Board and receives stock options from Leap Therapeutics, Dunad Therapeutics, Cygnal Therapeutics and Mestag Therapeutics outside the submitted work. D.A.T. has received unrelated research grant support from Fibrogen, Mestag and ONO Therapeutics. None of these activities are related to the current publication. R.L.L. is on the supervisory board of Qiagen and is a scientific advisor to Imago, Mission Bio, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics and Isoplexis. R.L.L. receives research support from Ajax, Zentalis and Abbvie and has consulted for Incyte, Janssen and AstraZeneca and has received honoraria from AstraZeneca for invited lectures. L.W.J. owns stock in Pacyclex and Illuminosonics. The other authors declare no competing interests.
Figures

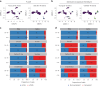
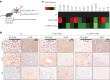
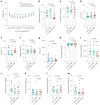
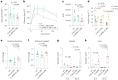


Update of
-
Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction.Res Sq [Preprint]. 2023 Sep 26:rs.3.rs-3385749. doi: 10.21203/rs.3.rs-3385749/v1. Res Sq. 2023. Update in: Nat Aging. 2024 Mar;4(3):336-349. doi: 10.1038/s43587-023-00560-5. PMID: 37841853 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
- R01 CA190092/CA/NCI NIH HHS/United States
- DP5 OD033055/OD/NIH HHS/United States
- U01 CA224013/CA/NCI NIH HHS/United States
- R35 CA197594/CA/NCI NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- U01 AG077921/AG/NIA NIH HHS/United States
- R01 CA188134/CA/NCI NIH HHS/United States
- R01 AG065396/AG/NIA NIH HHS/United States
- R01 CA229699/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 AG082800/AG/NIA NIH HHS/United States
- U01 AG077925/AG/NIA NIH HHS/United States
- S10 OD028632/OD/NIH HHS/United States
- U01 CA210240/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

