Time is myelin: early cortical myelin repair prevents atrophy and clinical progression in multiple sclerosis
- PMID: 38267729
- PMCID: PMC10994569
- DOI: 10.1093/brain/awae024
Time is myelin: early cortical myelin repair prevents atrophy and clinical progression in multiple sclerosis
Abstract
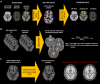
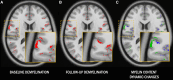
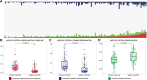
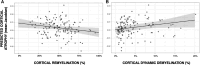
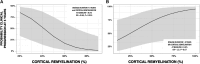
Cortical myelin loss and repair in multiple sclerosis (MS) have been explored in neuropathological studies, but the impact of these processes on neurodegeneration and the irreversible clinical progression of the disease remains unknown. Here, we evaluated in vivo cortical demyelination and remyelination in a large cohort of people with all clinical phenotypes of MS followed up for 5 years using magnetization transfer imaging (MTI), a technique that has been shown to be sensitive to myelin content changes in the cortex. We investigated 140 people with MS (37 clinically isolated syndrome, 71 relapsing-MS, 32 progressive-MS), who were clinically assessed at baseline and after 5 years and, along with 84 healthy controls, underwent a 3 T-MRI protocol including MTI at baseline and after 1 year. Changes in cortical volume over the radiological follow-up were computed with a Jacobian integration method. Magnetization transfer ratio was employed to calculate for each patient an index of cortical demyelination at baseline and of dynamic cortical demyelination and remyelination over the follow-up period. The three indices of cortical myelin content change were heterogeneous across patients but did not significantly differ across clinical phenotypes or treatment groups. Cortical remyelination, which tended to fail in the regions closer to CSF (-11%, P < 0.001), was extensive in half of the cohort and occurred independently of age, disease duration and clinical phenotype. Higher indices of cortical dynamic demyelination (β = 0.23, P = 0.024) and lower indices of cortical remyelination (β = -0.18, P = 0.03) were significantly associated with greater cortical atrophy after 1 year, independently of age and MS phenotype. While the extent of cortical demyelination predicted a higher probability of clinical progression after 5 years in the entire cohort [odds ratio (OR) = 1.2; P = 0.043], the impact of cortical remyelination in reducing the risk of accumulating clinical disability after 5 years was significant only in the subgroup of patients with shorter disease duration and limited extent of demyelination in cortical regions (OR = 0.86, P = 0.015, area under the curve = 0.93). In this subgroup, a 30% increase in cortical remyelination nearly halved the risk of clinical progression at 5 years, independently of clinical relapses. Overall, our results highlight the critical role of cortical myelin dynamics in the cascade of events leading to neurodegeneration and to the subsequent accumulation of irreversible disability in MS. Our findings suggest that early-stage myelin repair compensating for cortical myelin loss has the potential to prevent neuro-axonal loss and its long-term irreversible clinical consequences in people with MS.
Keywords: imaging; multiple sclerosis; myelin; neurodegeneration; neuroprotection; remyelination.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
A.L. has received funding for travel and speaker honoraria from Merck Serono, Roche, Novartis and Sandoz. None related to the present work. M.H. has nothing to disclose. M.T. is an employee of and owns stocks or stock options in F. Hoffmann-La Roche Ltd. A.-L.D. has nothing to disclose. M.K. has received speaker honoraria from Bayer, Novartis, Merck, Biogen Idec and Teva Pharmaceutical Industries Ltd. and serves on scientific advisory boards for Biogen Idec, Merck Serono, Roche, Novartis, Bristol-Myers Squibb and Gilead. He received research grants from Biogen and Novartis. L.P. has nothing to disclose. S.R. has received honoraria from Axon Neuroscience, QPS, and NeuroScios for consulting services. C.E. has received funding for travel and speaker honoraria from Biogen, Bayer Schering, Merck Serono, Novartis, Shire, Genzyme and Teva Pharmaceutical Industries Ltd., Sanofi-Aventis; research support from Merck Serono, Biogen, and Teva Pharmaceutical Industries Ltd./Sanofi-Aventis; and has served on scientific advisory boards for Bayer Schering, Biogen, Celgene, Merck Serono, Novartis, Roche and Teva Pharmaceutical Industries Ltd./Sanofi-Aventis. M.B. has nothing to disclose. M.L.S. has nothing to disclose. N.D. has received honoraria from Biogen-Idec, Bristol Myers Squibb, Celgene, Genzyme, Immunic, Merck Serono, Novartis, Roche and Teva for consulting services, speaking and travel support. He serves on advisory boards for Merck, Novartis, Biogen-Idec, Roche and Genzyme, Immunic and he has received research grant support from the Italian MS Society. M.F. is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Italian Ministry of Health, and Fondazione Italiana Sclerosi Multipla. M.A.R. received consulting fees from Biogen, Bristol Myers Squibb, Eli Lilly, Janssen, Roche; and speaker honoraria from AstraZaneca, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Genzyme, Horizon Therapeutics Italy, Merck Serono SpA, Novartis, Roche, Sanofi and Teva. She receives research support from the MS Society of Canada, the Italian Ministry of Health, and Fondazione Italiana Sclerosi Multipla. She is Associate Editor for Multiple Sclerosis and Related Disorders. P.G. has received grants and personal fees from Merck Serono, Biogen Idec, Genzyme Sanofi, grants and personal fees from Novartis, grants from University of Padua, Department of Neurosciences DNS, grants from Veneto Region of Italy, grants from Italian Association for Multiple Sclerosis (AISM), grants from Italian Ministry of Public Health, during the conduct of the study. C.G. has received fees as invited speaker or travel expenses for attending meeting from Biogen, Merck Serono, Teva, Mylan, Sanofi, Novartis, Genzyme, none related to the present work. B.S. has received grants and personal fees for lectures from Roche, Sanofi-Genzyme, Merck-Serono and Janssen, personal fees for lectures from Novartis, Biogen and Teva, all outside of the submitted work. B.B. has received funding for travel and speaker honoraria from Novartis, Genzyme, Roche and Merck Serono, and she received research support from Biogen, none related to the present work.
Figures
Comment in
-
Cortical remyelination in multiple sclerosis: a target for disease monitoring and intervention.Brain. 2024 Apr 4;147(4):1124-1126. doi: 10.1093/brain/awae083. Brain. 2024. PMID: 38537261 No abstract available.
References
-
- Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;214:183–193. - PubMed
-
- Lubetzki C, Zalc B, Williams A, Stadelmann C, Stankoff B. Remyelination in multiple sclerosis: From basic science to clinical translation. Lancet Neurol. 2020;19:678–688. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

