Anti-hyperalgesic and anti-inflammatory effects of 4R-tobacco cembranoid in a mouse model of inflammatory pain
- PMID: 38267952
- PMCID: PMC10809744
- DOI: 10.1186/s12950-023-00373-8
Anti-hyperalgesic and anti-inflammatory effects of 4R-tobacco cembranoid in a mouse model of inflammatory pain
Abstract
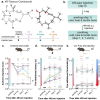
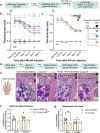
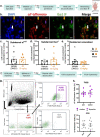
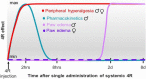
4R is a tobacco cembranoid that binds to and modulates cholinergic receptors and exhibits neuroprotective and anti-inflammatory activity. Given the established function of the cholinergic system in pain and inflammation, we propose that 4R is also analgesic. Here, we tested the hypothesis that systemic 4R treatment decreases pain-related behaviors and peripheral inflammation via modulation of the alpha 7 nicotinic acetylcholine receptors (α7 nAChRs) in a mouse model of inflammatory pain. We elicited inflammation by injecting Complete Freund's Adjuvant (CFA) into the hind paw of male and female mice. We then assessed inflammation-induced hypersensitivity to cold, heat, and tactile stimulation using the Acetone, Hargreaves, and von Frey tests, respectively, before and at different time points (2.5 h - 8d) after a single systemic 4R (or vehicle) administration. We evaluated the contribution of α7 nAChRs 4R-mediated analgesia by pre-treating mice with a selective antagonist of α7 nAChRs followed by 4R (or vehicle) administration prior to behavioral tests. We assessed CFA-induced paw edema and inflammation by measuring paw thickness and quantifying immune cell infiltration in the injected hind paw using hematoxylin and eosin staining. Lastly, we performed immunohistochemical and flow cytometric analyses of paw skin in α7 nAChR-cre::Ai9 mice to measure the expression of α7 nAChRs on immune subsets. Our experiments show that systemic administration of 4R decreases inflammation-induced peripheral hypersensitivity in male and female mice and inflammation-induced paw edema in male but not female mice. Notably, 4R-mediated analgesia and anti-inflammatory effects lasted up to 8d after a single systemic administration on day 1. Pretreatment with an α7 nAChR-selective antagonist prevented 4R-mediated analgesia and anti-inflammatory effects, demonstrating that 4R effects are via modulation of α7 nAChRs. We further show that a subset of immune cells in the hind paw expresses α7 nAChRs. However, the number of α7 nAChR-expressing immune cells is unaltered by CFA or 4R treatment, suggesting that 4R effects are independent of α7 nAChR-expressing immune cells. Together, our findings identify a novel function of the 4R tobacco cembranoid as an analgesic agent in both male and female mice that reduces peripheral inflammation in a sex-dependent manner, further supporting the pharmacological targeting of the cholinergic system for pain treatment.
Keywords: 4R; Hyperalgesia; Inflammatory pain; Macrophage; Paw edema; Persistent analgesia; Tobacco cembranoid; α7 nicotinic acetylcholine receptors.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Berlowitz I, Torres EG, Walt H, Wolf U, Maake C, Martin-Soelch C. "Tobacco Is the Chief Medicinal Plant in My Work": Therapeutic Uses of Tobacco in Peruvian Amazonian Medicine Exemplified by the Work of a Maestro Tabaquero. Front Pharmacol. 2020;11:594591. doi: 10.3389/fphar.2020.594591. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

