MiR-31-5p regulates the neuroinflammatory response via TRAF6 in neuropathic pain
- PMID: 38267979
- PMCID: PMC10807213
- DOI: 10.1186/s13062-023-00434-1
MiR-31-5p regulates the neuroinflammatory response via TRAF6 in neuropathic pain
Abstract
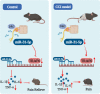
Background: Neuropathic pain is chronic pain and has few effective control strategies. Studies have demonstrated that microRNAs have functions in neuropathic pain. However, no study has been conducted to demonstrate the role and mechanism of microRNA (miR)-31-5p in neuropathic pain. Accordingly, this study sought to determine the pathological role of miR-31-5p in chronic constriction injury (CCI) -induced neuropathic pain mouse models.
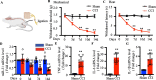
Methods: We used CCI surgery to establish mouse neuropathic pain model. Behavioral tests were performed to evaluate pain sensitivity of mice. Expressions of miR-31-5p and inflammatory cytokines in dorsal root ganglion (DRG) were examined by polymerase chain reaction. Animals or cells were received with/without miR-31-5p mimic or inhibitor to investigate its role in neuropathic pain. The mechanism of miR-31-5p was assayed using western blotting, immunofluorescence staining and dual-luciferase reporter assay.
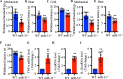
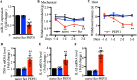
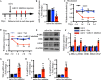
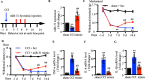
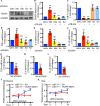
Results: We found that CCI led to a significant decrease in miR-31-5p levels. Knockout of miR-31-5p and administration of miPEP31 exacerbated pain in C57BL/6 mice. Meanwhile, miR-31-5p overexpression increased the paw withdrawal threshold and latency. TRAF6 is one of the target gene of miR-31-5p, which can trigger a complex inflammatory response. TRAF6 was associated with pain and that reducing the DRG expression of TRAF6 could alleviate pain. In addition, miR-31-5p overexpression inhibited the TRAF6 expression and reduced the neuroinflammatory response.
Conclusions: All the results reveal that miR-31-5p could potentially alleviate pain in CCI mouse models by inhibiting the TRAF6 mediated neuroinflammatory response. MiR-31-5p upregulation is highlighted here as new target for CCI treatment.
Keywords: Neuroinflammatory response; Neuropathic pain; TRAF6; miR-31-5p.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

