Selenium protects against nesfatin-1 modulation of the hypothalamic-pituitary-testicular axis during hypothyroidism in male rats
- PMID: 38268116
- PMCID: PMC10808778
- DOI: 10.14814/phy2.15923
Selenium protects against nesfatin-1 modulation of the hypothalamic-pituitary-testicular axis during hypothyroidism in male rats
Abstract
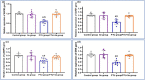
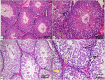
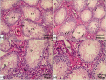
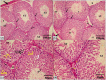
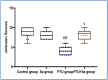
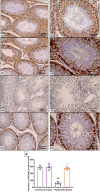
Normal gonadal function can be disrupted by hypothyroidism. Hypothyroidism disturbs testicular function directly and centrally by affecting the hypothalamic-pituitary-testicular axis with unclear mechanism. As nesfatin-1 neurons co-localized with TRH and GnRH neurons in the hypothalamus, it could play a role in centrally hypothyroidism induced testicular dysfunction. Selenium (Se), by affecting thyroid iodide supply, could relieve these disturbances. So, we aim to identify the role of nesfatin-1 as a link between testicular dysfunction and hypothyroidism through modulating the MAPK/ERK pathway while discussing the possible role of Se in alleviating hypothyroidism and associated testicular damage. Forty male rats were divided equally into: Control: distilled water, Se: Se orally, Propylthiouracil (PTU): PTU orally, PTU + Se: Se with PTU orally. Serum thyroid function, gonadal hormones, nesfatin-1, testicular redox status, sperm analysis, brain tissue GnRH, nucleobindin 2-derived polypeptide, pMAPK/ERK gene expression, histological changes and immunohistochemical expression of testicular proliferating cell antigen (PCNA) were done. PTU induced hypothyroidism and reduction of gonadal hormones which both were correlated with reduced nesfatin-1. There was testicular stress with reduced GnRH, NUCB2, pMAPK/ERK gene expression, and PCNA immunopositive cells. These parameters were reversed by Se. Nesfatin-1 could be the central link between hypothyroidism and disturbances of the hypothalamic pituitary testicular axis.
Keywords: hypothalamic-pituitary-testicular axis; hypothyroidism; nesfatin-1; propylthiouracil; selenium.
© 2024 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors did not disclose any potential conflicts of interest.
Figures


References
-
- Algaidi, S. A. , Faddladdeen, K. A. , Alrefaei, G. I. , Qahl, S. H. , Albadawi, E. A. , ALmohaimeed, H. M. , & Ayuob, N. N. (2022). Thymoquinone protects the testes of hypothyroid rats by suppressing pro‐inflammatory cytokines and oxidative stress and promoting SIRT1 testicular expression. Frontiers in Pharmacology, 13, 4873. 10.3389/fphar.2022.1040857 - DOI - PMC - PubMed
-
- Alkalby, J. M. , & Alzerjawi, S. J. (2013). Effect of propylthiouracil‐induced hypothyroidism on reproductive efficiency of adult male rats. Journal of Veterinary Research, 12(2), 113–121. 10.1007/BF02537073 - DOI
-
- Angelone, T. , Filice, E. , Pasqua, T. , Amodio, N. , Galluccio, M. , Montesanti, G. , Quintieri, A. M. , & Cerra, M. C. (2013). Nesfatin‐1 as a novel cardiac peptide: Identification, functional characterization, and protection against ischemia/reperfusion injury. Cellular and Molecular Life Sciences, 70, 495–509. 10.1007/s00018-012-1138-7 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

