Avacopan for ANCA-associated vasculitis with hypoxic pulmonary haemorrhage
- PMID: 38268434
- PMCID: PMC11361805
- DOI: 10.1093/ndt/gfae020
Avacopan for ANCA-associated vasculitis with hypoxic pulmonary haemorrhage
Abstract
Background: Pulmonary haemorrhage with hypoxia caused by anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) has a high early mortality. Avacopan, an oral C5a receptor antagonist, is an approved treatment for AAV, but patients with pulmonary haemorrhage requiring invasive pulmonary ventilation support were excluded from the Avacopan for the Treatment of ANCA-Associated Vasculitis (ADVOCATE) Trial.
Methods: A retrospective, observational, multicentre case series of AAV patients with hypoxic pulmonary haemorrhage, requiring oxygen support or mechanical ventilation, who received avacopan.
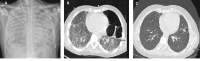
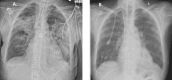
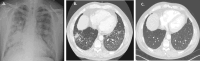
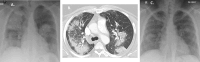
Results: Eight patients (62.5% female), median age 64 years (range 17-80), seven with kidney involvement, median estimated glomerular filtration rate (eGFR) 11 (range 5-99) mL/min/1.73 m2, were followed for a median of 6 months from presentation. Seven were newly diagnosed (87.5%), five were myeloperoxidase-ANCA and three proteinase 3-ANCA positive. All had hypoxia, four requiring mechanical ventilation (three invasive and one non-invasive). Intensive care unit (ICU) stay for the four patients lasted a median of 9 days (range 6-60). Four received rituximab and cyclophosphamide combination, three rituximab and one cyclophosphamide. Four underwent plasma exchange and one received 2 months of daily extracorporeal membrane oxygenation therapy. Following the initiation of avacopan after a median of 10 days (range 2-40), pulmonary haemorrhage resolved in all patients, even the two who had 1 month of refractory pulmonary haemorrhage prior to avacopan. Additionally, after 1 month, the median prednisolone dose was 5 mg/day (range 0-50), with three patients successfully discontinuing steroid use. Two patients suffered serious infections, two discontinued avacopan, one permanently due to a rash and one temporarily after 3 months due to neutropenia. All patients survived and no re-hospitalization occurred.
Conclusion: We report the use of avacopan as a component of the treatment for pulmonary haemorrhage with hypoxia in AAV. Despite the life-threatening presentations all patients recovered, but attribution of the positive outcomes to avacopan is limited by the concomitant therapies and retrospective observational design.
Keywords: ANCA; avacopan; hypoxia; pulmonary haemorrhage; vasculitis.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
O.F. has received lecture or consulting fees from Vifor. R.J. has received research grants from Roche, GSK, fees or honoraria from Roche and Vifor. T.S. received fees from Vifor. L.W. has received lecture fees from Otsuka. D.J. has received consulting fees from AstraZeneca, CSL Vifor, GSK, Novartis, Roche and Takeda, lecture fees from CSL Vifor, Amgen and GSK and research grants from GSK, Roche and CSL Vifor. The other authors declared no conflicts of interest related to this work.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

