A paradoxical switch: the implications of excitatory GABAergic signaling in neurological disorders
- PMID: 38268565
- PMCID: PMC10805837
- DOI: 10.3389/fpsyt.2023.1296527
A paradoxical switch: the implications of excitatory GABAergic signaling in neurological disorders
Abstract
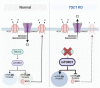
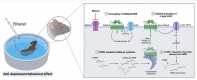
Gamma-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the central nervous system. In the mature brain, inhibitory GABAergic signaling is critical in maintaining neuronal homeostasis and vital human behaviors such as cognition, emotion, and motivation. While classically known to inhibit neuronal function under physiological conditions, previous research indicates a paradoxical switch from inhibitory to excitatory GABAergic signaling that is implicated in several neurological disorders. Various mechanisms have been proposed to contribute to the excitatory switch such as chloride ion dyshomeostasis, alterations in inhibitory receptor expression, and modifications in GABAergic synaptic plasticity. Of note, the hypothesized mechanisms underlying excitatory GABAergic signaling are highlighted in a number of neurodevelopmental, substance use, stress, and neurodegenerative disorders. Herein, we present an updated review discussing the presence of excitatory GABAergic signaling in various neurological disorders, and their potential contributions towards disease pathology.
Keywords: GABA; GABAARs; depolarizing; diseases; excitatory; neurological.
Copyright © 2024 McArdle, Arnone, Heaney and Raab-Graham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





Similar articles
-
Depolarizing, inhibitory GABA type A receptor activity regulates GABAergic synapse plasticity via ERK and BDNF signaling.Neuropharmacology. 2018 Jan;128:324-339. doi: 10.1016/j.neuropharm.2017.10.022. Epub 2017 Oct 23. Neuropharmacology. 2018. PMID: 29074304 Free PMC article.
-
The Alteration of Chloride Homeostasis/GABAergic Signaling in Brain Disorders: Could Oxidative Stress Play a Role?Antioxidants (Basel). 2021 Aug 21;10(8):1316. doi: 10.3390/antiox10081316. Antioxidants (Basel). 2021. PMID: 34439564 Free PMC article. Review.
-
The basolateral amygdala γ-aminobutyric acidergic system in health and disease.J Neurosci Res. 2016 Jun;94(6):548-67. doi: 10.1002/jnr.23690. Epub 2015 Nov 19. J Neurosci Res. 2016. PMID: 26586374 Free PMC article. Review.
-
Use-dependent shift from inhibitory to excitatory GABAA receptor action in SP-O interneurons in the rat hippocampal CA3 area.J Neurophysiol. 2003 Sep;90(3):1983-95. doi: 10.1152/jn.00060.2003. Epub 2003 May 15. J Neurophysiol. 2003. PMID: 12750426
-
The ketone body β-hydroxybutyrate ameliorates neurodevelopmental deficits in the GABAergic system of daf-18/PTEN Caenorhabditis elegans mutants.Elife. 2024 Oct 18;13:RP94520. doi: 10.7554/eLife.94520. Elife. 2024. PMID: 39422188 Free PMC article.
Cited by
-
Gut microbiota-mediated pain sensitization: mechanisms and therapeutic implications.Front Pain Res (Lausanne). 2025 Jul 3;6:1626515. doi: 10.3389/fpain.2025.1626515. eCollection 2025. Front Pain Res (Lausanne). 2025. PMID: 40678181 Free PMC article. Review.
-
Neonatal Procedural Pain Disrupts Phosphorylation of KCC2 in the Spinal Cord.Dev Neurobiol. 2025 Oct;85(4):e22993. doi: 10.1002/dneu.22993. Dev Neurobiol. 2025. PMID: 40790923 Free PMC article.
-
Development of KCC2 therapeutics to treat neurological disorders.Front Mol Neurosci. 2024 Dec 10;17:1503070. doi: 10.3389/fnmol.2024.1503070. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39720463 Free PMC article.
-
A novel de novo GABRA2 gene missense variant causing developmental epileptic encephalopathy in a Chinese patient.Ann Clin Transl Neurol. 2025 Jan;12(1):137-148. doi: 10.1002/acn3.52262. Epub 2024 Dec 31. Ann Clin Transl Neurol. 2025. PMID: 39737842 Free PMC article.
-
Gestational Duration and Postnatal Age-Related Changes in Aperiodic and Periodic Parameters in Neonatal and Toddler Electroencephalogram (EEG).Hum Brain Mapp. 2025 Jan;46(1):e70130. doi: 10.1002/hbm.70130. Hum Brain Mapp. 2025. PMID: 39764646 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

