Molecular epidemiology study of programmed death ligand 1 and ligand 2 protein expression assessed by immunohistochemistry in extensive-stage small-cell lung cancer
- PMID: 38269020
- PMCID: PMC10807038
- DOI: 10.3389/fonc.2023.1225820
Molecular epidemiology study of programmed death ligand 1 and ligand 2 protein expression assessed by immunohistochemistry in extensive-stage small-cell lung cancer
Abstract
Objectives: Prevalence of tumor PD-L1 expression in extensive-stage small-cell lung cancer (ES-SCLC) is variable, and data on PD-L2 expression are limited. The prognostic values of these biomarkers are not well understood. The current study was conducted to address these data gaps.
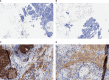
Methods: A retrospective cohort study of Danish patients with histologically confirmed ES-SCLC and evaluable tumor samples who were receiving usual care before the introduction of immunotherapy was conducted. Protein expression of PD-L1 and PD-L2 was determined by immunohistochemistry (IHC) using the PD-L1 IHC 22C3 pharmDx assay and a PD-L2 IHC assay using a propriety mouse monoclonal antibody. A combined positive score (CPS) of ≥1 was used to define biomarker positivity. Kaplan-Meier plots and Cox proportional hazard models were employed to assess the relationship between PD-L1 and PD-L2 protein expression and OS.
Results: Among 80 patients, 31% (n=25) and 36% (n=29) had disease positive for PD-L1 and PD-L2, respectively. Overall, 85% (n=68) of patients had concordant PD-L1/PD-L2 status; 26% (n=21) had double positive disease (both PD-L1 and PD-L2 CPS ≥1) and 59% (n=47) had double negative disease (both PD-L1 and PD-L2 CPS <1). PD-L1 and PD-L2 positivity were each associated with longer OS (unadjusted hazard ratios [HRs], 0.35 [95% CI, 0.21-0.61] and 0.50 [95% CI, 0.31-0.82]); the associations persisted after adjustment for several known prognostic factors (HRs, 0.41 [95% CI, 0.22-0.75] and 0.44 [95% CI, 0.25-0.79] for PD-L1 and PD-L2 positivity, respectively). When evaluating OS in patients with double positive disease, unadjusted and adjusted HRs for double positive compared with double negative were similar to those with only PD-L1 or PD-L2 positivity (unadjusted HR, 0.36 [95% CI, 0.20-0.64]; adjusted HR, 0.36 [0.18-0.73]).
Conclusion: PD-L1 and PD-L2 positivity were observed in approximately one-third of assessed ES-SCLC tumor samples and were highly congruent. Patients with PD-L1 and PD-L2 positivity, alone or combined, were associated with longer OS, independent of other prognostic factors.
Keywords: PD-L1; PD-L2; biomarker; extensive-stage small-cell lung cancer; prevalence.
Copyright © 2024 Steiniche, Georgsen, Meldgaard, Deitz, Ayers, Pietanza and Zu.
Conflict of interest statement
TS: Employee of Aarhus University Hospital, who received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, to conduct this study. Fiduciary role for the Danish Medicines Council as a member of a subgroup concerning targeted treatment of cancer regardless of the histological type. JBG: Employee of Aarhus University Hospital, Region Midt, Denmark, who received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, to conduct this study PM: Employee of Aarhus University Hospital, who received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, to conduct this study. KZ, ACD, MA, and MCP: Employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and stockholders of Merck & Co., Inc., Rahway, NJ, USA.
Figures
Similar articles
-
Accurate expression of PD-L1/L2 in lung adenocarcinoma cells: A retrospective study by double immunohistochemistry.Cancer Sci. 2019 Sep;110(9):2711-2721. doi: 10.1111/cas.14128. Epub 2019 Jul 31. Cancer Sci. 2019. PMID: 31294893 Free PMC article.
-
Clinical significance of programmed cell death-ligand expression in small bowel adenocarcinoma is determined by the tumor microenvironment.World J Gastroenterol. 2023 Oct 28;29(40):5566-5581. doi: 10.3748/wjg.v29.i40.5566. World J Gastroenterol. 2023. PMID: 37970475 Free PMC article.
-
Prognostic Impact of PD-L2 Expression and Association with PD-L1 in Patients with Small-cell Lung Cancer.Anticancer Res. 2018 Oct;38(10):5903-5907. doi: 10.21873/anticanres.12934. Anticancer Res. 2018. PMID: 30275217
-
Efficacy and safety of PD-1/PD-L1 inhibitor plus chemotherapy versus chemotherapy alone as first-line treatment for extensive-stage small cell lung cancer: A systematic review and meta-analysis.Thorac Cancer. 2020 Dec;11(12):3536-3546. doi: 10.1111/1759-7714.13698. Epub 2020 Oct 15. Thorac Cancer. 2020. PMID: 33058504 Free PMC article.
-
Predictive Values of Programmed Cell Death-Ligand 1 Expression for Prognosis, Clinicopathological Factors, and Response to Programmed Cell Death-1/Programmed Cell Death-Ligand 1 Inhibitors in Patients With Gynecological Cancers: A Meta-Analysis.Front Oncol. 2021 Feb 1;10:572203. doi: 10.3389/fonc.2020.572203. eCollection 2020. Front Oncol. 2021. PMID: 33634012 Free PMC article.
References
-
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. . Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol (2006) 24:4539–44. doi: 10.1200/JCO.2005.04.4859 - DOI - PubMed
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Small Cell Lung Cancer Version 2.2023. (2022) Plymouth Meeting, PA, USA: NCCN.
LinkOut - more resources
Full Text Sources
Research Materials

