Metal-Mediated Catalytic Polarization Transfer from para Hydrogen to 3,5-Dihalogenated Pyridines
- PMID: 38269038
- PMCID: PMC10804365
- DOI: 10.1021/acscatal.3c05378
Metal-Mediated Catalytic Polarization Transfer from para Hydrogen to 3,5-Dihalogenated Pyridines
Abstract
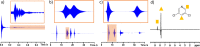
The neutral catalysts [IrCl(H)2(NHC)(substrate)2] or [IrCl(H)2(NHC)(substrate)(sulfoxide)] are used to transfer polarization from para hydrogen (pH2) to 3,5-dichloropyridine and 3,5-dibromopyridine substrates. This is achieved in a rapid, reversible, and low-cost process that relies on ligand exchange within the active catalyst. Notably, the sulfoxide-containing catalyst systems produced NMR signal enhancements between 1 and 2 orders of magnitude larger than its unmodified counterpart. Consequently, this signal amplification by reversible exchange hyperpolarization method can boost the 1H, 13C, and 15N nuclear magnetic resonance (NMR) signal intensities by factors up to 4350, 1550, and 46,600, respectively (14.0, 1.3, and 15.4% polarization). In this paper, NMR and X-ray crystallography are used to map the evolution of catalytically important species and provide mechanistic rational for catalytic efficiency. Furthermore, applications in spontaneous radiofrequency amplification by stimulated emission and NMR reaction monitoring are also shown.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Schmidt A. B.; Bowers C. R.; Buckenmaier K.; Chekmenev E. Y.; de Maissin H.; Eills J.; Ellermann F.; Glöggler S.; Gordon J. W.; Knecht S.; et al. Instrumentation for Hydrogenative Parahydrogen-Based Hyperpolarization Techniques. Anal. Chem. 2022, 94 (1), 479–502. 10.1021/acs.analchem.1c04863. - DOI - PMC - PubMed
-
- Adams R. W.; Aguilar J. A.; Atkinson K. D.; Cowley M. J.; Elliott P. I. P.; Duckett S. B.; Green G. G. R.; Khazal I. G.; López-Serrano J.; Williamson D. C. Reversible Interactions with Para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323 (5922), 1708–1711. 10.1126/science.1168877. - DOI - PubMed
