Organocatalytic Asymmetric Synthesis of Si-Stereogenic Siloxanols
- PMID: 38269039
- PMCID: PMC10804373
- DOI: 10.1021/acscatal.3c03932
Organocatalytic Asymmetric Synthesis of Si-Stereogenic Siloxanols
Abstract
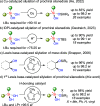
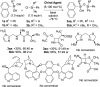
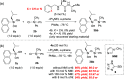
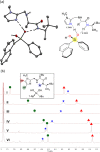
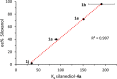
We report the organocatalytic synthesis of Si-stereogenic compounds via desymmetrization of a prochiral silanediol with a chiral imidazole-containing catalyst. This metal-free silylation method affords high yields with enantioselectivity up to 98:2 for various silanediol and silyl chloride substrate combinations (including secondary alkyl, vinyl, and H groups), accessing products with potential for further elaboration. NMR and X-ray studies reveal insight into the H-bonding interactions between the imidazole organocatalyst and the silanediol and the dual activating role of the Lewis basic imidazole to account for the high enantioselectivity.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
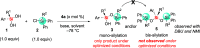
References
-
- Hiyama T.; Oestreich M. Organosilicon Chemistry: Novel Approaches and Reactions. Organosilicon Chem. Nov. Approaches React. 2019, 1–550. 10.1002/9783527814787. - DOI
-
- Rendler S.; Auer G.; Keller M.; Oestreich M. Preparation of a Privileged Silicon-Stereogenic Silane: Classical versus Kinetic Resolution. Adv. Synth. Catal. 2006, 348 (10–11), 1171–1182. 10.1002/adsc.200606071. - DOI
LinkOut - more resources
Full Text Sources
