Impaired communication at the neuromotor axis during Degenerative Cervical Myelopathy
- PMID: 38269114
- PMCID: PMC10806149
- DOI: 10.3389/fncel.2023.1316432
Impaired communication at the neuromotor axis during Degenerative Cervical Myelopathy
Abstract
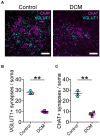
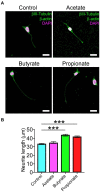
Degenerative Cervical Myelopathy (DCM) is a progressive neurological condition characterized by structural alterations in the cervical spine, resulting in compression of the spinal cord. While clinical manifestations of DCM are well-documented, numerous unanswered questions persist at the molecular and cellular levels. In this study, we sought to investigate the neuromotor axis during DCM. We use a clinically relevant mouse model, where after 3 months of DCM induction, the sensorimotor tests revealed a significant reduction in both locomotor activity and muscle strength compared to the control group. Immunohistochemical analyses showed alterations in the gross anatomy of the cervical spinal cord segment after DCM. These changes were concomitant with the loss of motoneurons and a decrease in the number of excitatory synaptic inputs within the spinal cord. Additionally, the DCM group exhibited a reduction in the endplate surface, which correlated with diminished presynaptic axon endings in the supraspinous muscles. Furthermore, the biceps brachii (BB) muscle exhibited signs of atrophy and impaired regenerative capacity, which inversely correlated with the transversal area of remnants of muscle fibers. Additionally, metabolic assessments in BB muscle indicated an increased proportion of oxidative skeletal muscle fibers. In line with the link between neuromotor disorders and gut alterations, DCM mice displayed smaller mucin granules in the mucosa layer without damage to the epithelial barrier in the colon. Notably, a shift in the abundance of microbiota phylum profiles reveals an elevated Firmicutes-to-Bacteroidetes ratio-a consistent hallmark of dysbiosis that correlates with alterations in gut microbiota-derived metabolites. Additionally, treatment with short-chain fatty acids stimulated the differentiation of the motoneuron-like NSC34 cell line. These findings shed light on the multifaceted nature of DCM, resembling a synaptopathy that disrupts cellular communication within the neuromotor axis while concurrently exerting influence on other systems. Notably, the colon emerges as a focal point, experiencing substantial perturbations in both mucosal barrier integrity and the delicate balance of intestinal microbiota.
Keywords: Degenerative Cervical Myelopathy; motoneuron; muscle; neuromuscular; spinal cord; synapse.
Copyright © 2024 Ojeda, Vergara, Ávila, Henríquez, Fehlings and Vidal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Degenerative Cervical Myelopathy induces sex-specific dysbiosis in mice.Front Microbiol. 2023 Oct 20;14:1229783. doi: 10.3389/fmicb.2023.1229783. eCollection 2023. Front Microbiol. 2023. PMID: 37928672 Free PMC article.
-
Subclinical respiratory dysfunction and impaired ventilatory adaptation in degenerative cervical myelopathy.Exp Neurol. 2024 Jan;371:114600. doi: 10.1016/j.expneurol.2023.114600. Epub 2023 Oct 29. Exp Neurol. 2024. PMID: 37907124
-
Degenerative cervical myelopathy: establishing severity thresholds for neuromotor dysfunction in the aging spine using the NIH Toolbox Assessment Scale.Geroscience. 2024 Apr;46(2):2197-2206. doi: 10.1007/s11357-023-00983-3. Epub 2023 Oct 25. Geroscience. 2024. PMID: 37880488 Free PMC article.
-
How Is Spinal Cord Function Measured in Degenerative Cervical Myelopathy? A Systematic Review.J Clin Med. 2022 Mar 5;11(5):1441. doi: 10.3390/jcm11051441. J Clin Med. 2022. PMID: 35268533 Free PMC article. Review.
-
Evidence-based commentary on the diagnosis, management, and further research of degenerative cervical spinal cord compression in the absence of clinical symptoms of myelopathy.Front Neurol. 2024 May 10;15:1341371. doi: 10.3389/fneur.2024.1341371. eCollection 2024. Front Neurol. 2024. PMID: 38798708 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

