In vivo evidence for GDP-fucose transport in the absence of transporter SLC35C1 and putative transporter SLC35C2
- PMID: 38270391
- PMCID: PMC10709068
- DOI: 10.1016/j.jbc.2023.105406
In vivo evidence for GDP-fucose transport in the absence of transporter SLC35C1 and putative transporter SLC35C2
Abstract
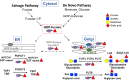
Slc35c1 encodes an antiporter that transports GDP-fucose into the Golgi and returns GMP to the cytoplasm. The closely related gene Slc35c2 encodes a putative GDP-fucose transporter and promotes Notch fucosylation and Notch signaling in cultured cells. Here, we show that HEK293T cells lacking SLC35C1 transferred reduced amounts of O-fucose to secreted epidermal growth factor-like repeats from NOTCH1 or secreted thrombospondin type I repeats from thrombospondin 1. However, cells lacking SLC35C2 did not exhibit reduced fucosylation of these epidermal growth factor-like repeats or thrombospondin type I repeats. To investigate SLC35C2 functions in vivo, WW6 embryonic stem cells were targeted for Slc35c2. Slc35c2[-/-] mice were viable and fertile and exhibited no evidence of defective Notch signaling during skeletal or T cell development. By contrast, mice with inactivated Slc35c1 exhibited perinatal lethality and marked skeletal defects in late embryogenesis, typical of defective Notch signaling. Compound Slc35c1[-/-]Slc35c2[-/-] mutants were indistinguishable in skeletal phenotype from Slc35c1[-/-] embryos and neonates. Double mutants did not exhibit the exacerbated skeletal defects predicted if SLC35C2 was functionally important for Notch signaling in vivo. In addition, NOTCH1 immunoprecipitated from Slc35c1[-/-]Slc35c2[-/-] neonatal lung carried fucose detected by binding of Aleuria aurantia lectin. Given that the absence of both SLC35C1, a known GDP-fucose transporter, and SLC35C2, a putative GDP-fucose transporter, did not lead to afucosylated NOTCH1 nor to the severe Notch signaling defects and embryonic lethality expected if all GDP-fucose transport were abrogated, at least one more mechanism of GDP-fucose transport into the secretory pathway must exist in mammals.
Keywords: GDP-fucose transporter; Notch signaling; O-fucose glycans; gene deletion; skeletal development.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Ginsburg V. Formation of guanosine diphosphate L-fucose from guanosine diphosphate D-mannose. J. Biol. Chem. 1960;235:2196–2201. - PubMed
-
- Yurchenco P.D., Atkinson P.H. Equilibration of fucosyl glycoprotein pools in HeLa cells. Biochemistry. 1977;16:944–953. - PubMed
-
- Ohyama C., Smith P.L., Angata K., Fukuda M.N., Lowe J.B., Fukuda M. Molecular cloning and expression of GDP-D-mannose-4,6-dehydratase, a key enzyme for fucose metabolism defective in Lec13 cells. J. Biol. Chem. 1998;273:14582–14587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

